Page 129 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioTechnology
P. 129
P1: GLQ/GUB P2: FYK Final Pages
Encyclopedia of Physical Science and Technology EN007K-319 July 2, 2001 17:53
Hybridomas, Genetic Engineering of 439
monoclonal antibodies as therapeutic agents. Any in vitro
production process results in a heterogeneity of glycan
structures of the product protein. To avoid any unde-
sirable immune response in the use of such antibodies
it is important to maximize the content of fully pro-
cessed glycans. There are various parameters that affect
the glycan processing from the metabolic profile of the
hybridoma to the environmental conditions of culture.
It has been shown that the glycan structures vary with
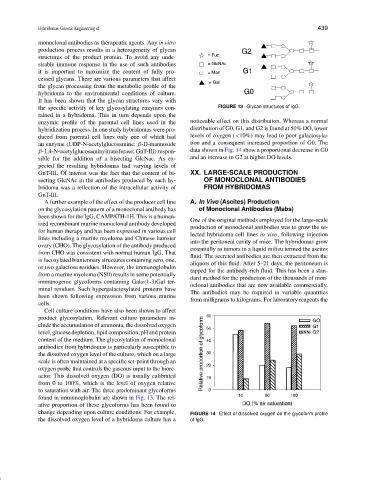
the specific activity of key glycosylating enzymes con- FIGURE 13 Glycan structures of IgG.
tained in a hybridoma. This in turn depends upon the
enzymic profile of the parental cell lines used in the noticeable effect on this distribution. Whereas a normal
hybridization process. In one study hybridomas were pro- distribution of G0, G1, and G2 is found at 50% DO, lower
duced from parental cell lines only one of which had levels of oxygen (<10%) may lead to poor galactosyla-
an enzyme (UDP-N-acetylglucosamine: β-D-mannoside tion and a consequent increased proportion of G0. The
β-1,4-N-acetylglucosaminyltransferase; GnT-III) respon- data shown in Fig. 14 show a proportional decrease in G0
sible for the addition of a bisecting GlcNac. As ex- and an increase in G2 at higher DO levels.
pected the resulting hybridomas had varying levels of
GnT-III. Of interest was the fact that the content of bi- XX. LARGE-SCALE PRODUCTION
secting GlcNAc in the antibodies produced by each hy- OF MONOCLONAL ANTIBODIES
bridoma was a reflection of the intracellular activity of FROM HYBRIDOMAS
GnT-III.
A further example of the effect of the producer cell line A. In Vivo (Ascites) Production
on the glycosylation pattern of a monoclonal antibody has of Monoclonal Antibodies (Mabs)
been shown for the IgG, CAMPATH-1H. This is a human-
One of the original methods employed for the large-scale
ized recombinant murine monoclonal antibody developed
production of monoclonal antibodies was to grow the se-
for human therapy and has been expressed in various cell
lected hybridoma cell lines in vivo, following injection
lines including a murine myeloma and Chinese hamster
into the peritoneal cavity of mice. The hybridomas grow
ovary (CHO). The glycosylation of the antibody produced
essentially as tumors in a liquid milieu termed the ascites
from CHO was consistent with normal human IgG. That
fluid. The secreted antibodies are then extracted from the
is fucosylated biantennary structures containing zero, one,
aliquots of this fluid. After 5–21 days, the peritoneum is
or two galactose residues. However, the immunoglobulin
tapped for the antibody-rich fluid. This has been a stan-
from a murine myeloma (NS0) results in some potentially
dard method for the production of the thousands of mon-
immunogenic glycoforms containing Galα(1-3)Gal ter-
oclonal antibodies that are now available commercially.
minal residues. Such hypergalactosylated proteins have
The antibodies may be required in variable quantities
been shown following expression from various murine
from milligrams to kilograms. For laboratory reagents the
cells.
Cell culture conditions have also been shown to affect
product glycosylation. Relevant culture parameters in-
clude the accumulation of ammonia, the dissolved oxygen
level, glucose depletion, lipid composition, pH and protein
content of the medium. The glycosylation of monoclonal
antibodies from hybridomas is particularly susceptible to
the dissolved oxygen level of the culture, which on a large
scale is often maintained at a specific set-point through an
oxygen probe that controls the gaseous input to the biore-
actor. This dissolved oxygen (DO) is usually calibrated
from 0 to 100%, which is the level of oxygen relative
to saturation with air. The three predominant glycoforms
found in immunoglobulin are shown in Fig. 13. The rel-
ative proportion of these glycoforms has been found to
change depending upon culture conditions. For example, FIGURE 14 Effect of dissolved oxygen on the glycoform profile
the dissolved oxygen level of a hybridoma culture has a of IgG.