Page 171 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioTechnology
P. 171
P1: GKY/GLQ/GTK P2: GLM Final Pages
Encyclopedia of Physical Science and Technology en009I-422 July 6, 2001 19:57
Metabolic Engineering 399
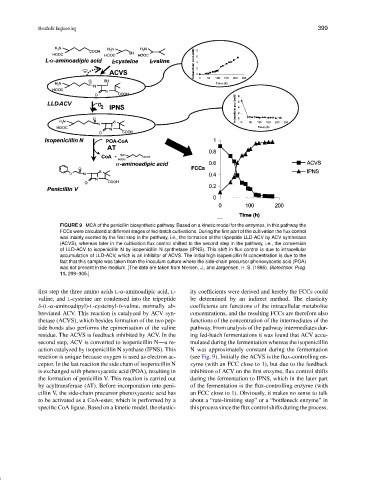
FIGURE 9 MCA of the penicillin biosynthetic pathway. Based on a kinetic model for the enzymes, in this pathway the
FCCs were calculated at different stages of fed-batch cultivations. During the first part of the cultivation the flux control
was mainly exerted by the first step in the pathway, i.e., the formation of the tripeptide LLD-ACV by ACV synthetase
(ACVS), whereas later in the cultivation flux control shifted to the second step in the pathway, i.e., the conversion
of LLD-ACV to isopenicillin N by isopenicillin N synthetase (IPNS). This shift in flux control is due to intracellular
accumulation of LLD-ACV, which is an inhibitor of ACVS. The initial high isopenicillin N concentration is due to the
fact that this sample was taken from the inoculum culture where the side-chain precursor phenoxyacetic acid (POA)
was not present in the medium. [The data are taken from Nielsen, J., and Jørgensen, H. S. (1995). Biotechnol. Prog.
11, 299–305.]
first step the three amino acids L-α-aminoadipic acid, L- ity coefficients were derived and hereby the FCCs could
valine, and L-cysteine are condensed into the tripeptide be determined by an indirect method. The elasticity
δ-(L-α-aminoadipyl)-L-cysteinyl-D-valine, normally ab- coefficients are functions of the intracellular metabolite
breviated ACV. This reaction is catalyzed by ACV syn- concentrations, and the resulting FCCs are therefore also
thetase (ACVS), which besides formation of the two pep- functions of the concentration of the intermediates of the
tide bonds also performs the epimerisation of the valine pathway. From analysis of the pathway intermediates dur-
residue. The ACVS is feedback inhibited by ACV. In the ing fed-batch fermentations it was found that ACV accu-
second step, ACV is converted to isopenicillin N—a re- mulated during the fermentation whereas the isopenicillin
action catalyzed by isopenicillin N synthase (IPNS). This N was approximately constant during the fermentation
reaction is unique because oxygen is used as electron ac- (see Fig. 9). Initially the ACVS is the flux-controlling en-
ceptor. In the last reaction the side chain of isopenicillin N zyme (with an FCC close to 1), but due to the feedback
is exchanged with phenoxyacetic acid (POA), resulting in inhibition of ACV on the first enzyme, flux control shifts
the formation of penicillin V. This reaction is carried out during the fermentation to IPNS, which in the later part
by acyltransferase (AT). Before incorporation into peni- of the fermentation is the flux-controlling enzyme (with
cillin V, the side-chain precursor phenoxyacetic acid has an FCC close to 1). Obviously, it makes no sense to talk
to be activated as a CoA-ester, which is performed by a about a “rate-limiting step” or a “bottleneck enzyme” in
specific CoA ligase. Based on a kinetic model, the elastic- this process since the flux control shifts during the process.