Page 182 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioTechnology
P. 182
P1: FMX Final Pages
Encyclopedia of Physical Science and Technology EN009J-69 July 19, 2001 22:50
682 Microanalytical Assays
produce an electric current which is proportional to the glucose
concentration. PO 2 is measured amperometrically. The oxygen
sensor is similar to a conventional Clark electrode. Oxygen per-
meates through a gas permeable membrane from the blood sam-
ple into an internal electrolyte solution where it is reduced at
the cathode. The oxygen reduction current is proportional to the
dissolved oxygen concentration. Hematocrit is determined con-
ductometrically. The measured conductivity, after correction for
electrolyte concentration, is related to the hematocrit. A variety
3
of calculated results are available that include HCO , TCO 2 , BE,
sO 2 , Anion Gap and Hemoglobin.
The concept of developing array technologies for mas-
sive analysis in biotechnology research was introduced in
1991. A group at Affymax showed that gene sequencing
and gene discovery could be accomplished by immobi-
lizing thousands of polynucleotide fragments of known
characteristics as a matrix of micron-sized spots on a
microscope-like slides. The array chip is then exposed to
fluorescently labeled gene fragments from a sample under
study. Hybridization is allowed to occur and then by the
pattern of fluorescent spots on the chip the DNA sequence
of the unknown sample can be deduced. Array technology
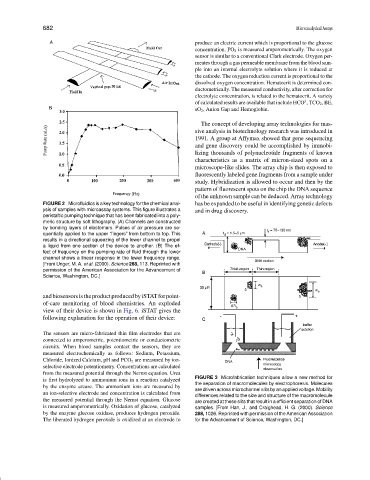
FIGURE 2 Microfluidics is a key technology for the chemical anal- has be expanded to be useful in identifying genetic defects
ysis of samples with microassay systems. This figure illustrates a and in drug discovery.
peristaltic pumping technique that has been fabricated into a poly-
meric structure by soft lithography. (A) Channels are constructed
by bonding layers of elastomers. Pulses of air pressure are se-
quentially applied to the upper “fingers” from bottom to top. This
results in a directional squeezing of the lower channel to propel
a liquid from one section of the device to another. (B) The ef-
fect of frequency on the pumping rate of fluid through the lower
channel shows a linear response in the lower frequency range.
[From Unger, M. A. et al. (2000). Science 288, 113. Reprinted with
permission of the American Association for the Advancement of
Science, Washington, DC.]
andbiosensorsistheproductproducedbyiSTATforpoint-
of-care monitoring of blood chemistries. An exploded
view of their device is shown in Fig. 6. iSTAT gives the
following explanation for the operation of their device:
The sensors are micro-fabricated thin film electrodes that are
connected to amperometric, potentiometric or conductometric
circuits. When blood samples contact the sensors, they are
measured electrochemically as follows: Sodium, Potassium,
Chloride, Ionized Calcium, pH and PCO 2 are measured by ion-
selective electrode potentiometry. Concentrations are calculated
from the measured potential through the Nernst equation. Urea
FIGURE 3 Microfabrication techniques allow a new method for
is first hydrolyzed to ammonium ions in a reaction catalyzed
the separation of macromolecules by electrophoresis. Molecules
by the enzyme urease. The ammonium ions are measured by
are driven across microchannel slits by an applied voltage. Mobility
an ion-selective electrode and concentration is calculated from
differences related to the size and structure of the macromolecule
the measured potential through the Nernst equation. Glucose are created at these slits that result in a efficient separation of DNA
is measured amperometrically. Oxidation of glucose, catalyzed samples. [From Han, J., and Craighead, H. G. (2000). Science
by the enzyme glucose oxidase, produces hydrogen peroxide. 288, 1026. Reprinted with permission of the American Association
The liberated hydrogen peroxide is oxidized at an electrode to for the Advancement of Science, Washington, DC.]