Page 285 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioTechnology
P. 285
P1: GPB/GRB P2: GLQ Final pages
Encyclopedia of Physical Science and Technology EN016J-783 August 1, 2001 10:58
836 Tissue Engineering
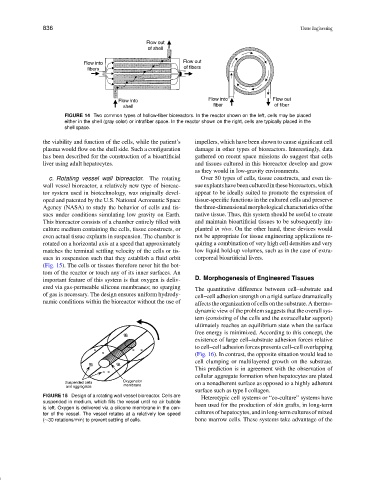
FIGURE 14 Two common types of hollow-fiber bioreactors. In the reactor shown on the left, cells may be placed
either in the shell (gray color) or intrafiber space. In the reactor shown on the right, cells are typically placed in the
shell space.
the viability and function of the cells, while the patient’s impellers, which have been shown to cause significant cell
plasma would flow on the shell side. Such a configuration damage in other types of bioreactors. Interestingly, data
has been described for the construction of a bioartificial gathered on recent space missions do suggest that cells
liver using adult hepatocytes. and tissues cultured in this bioreactor develop and grow
as they would in low-gravity environments.
c. Rotating vessel wall bioreactor. The rotating Over 50 types of cells, tissue constructs, and even tis-
wall vessel bioreactor, a relatively new type of bioreac- sueexplantshavebeenculturedinthesebioreactors,which
tor system used in biotechnology, was originally devel- appear to be ideally suited to promote the expression of
oped and patented by the U.S. National Aeronautic Space tissue-specific functions in the cultured cells and preserve
Agency (NASA) to study the behavior of cells and tis- the three-dimensional morphological characteristicsofthe
sues under conditions simulating low gravity on Earth. native tissue. Thus, this system should be useful to create
This bioreactor consists of a chamber entirely filled with and maintain bioartificial tissues to be subsequently im-
culture medium containing the cells, tissue constructs, or planted in vivo. On the other hand, these devices would
even actual tissue explants in suspension. The chamber is not be appropriate for tissue engineering applications re-
rotated on a horizontal axis at a speed that approximately quiring a combination of very high cell densities and very
matches the terminal settling velocity of the cells or tis- low liquid hold-up volumes, such as in the case of extra-
sues in suspension such that they establish a fluid orbit corporeal bioartificial livers.
(Fig. 15). The cells or tissues therefore never hit the bot-
tom of the reactor or touch any of its inner surfaces. An
important feature of this system is that oxygen is deliv- D. Morphogenesis of Engineered Tissues
ered via gas-permeable silicone membranes; no sparging The quantitative difference between cell–substrate and
of gas is necessary. The design ensures uniform hydrody- cell–cell adhesion strength on a rigid surface dramatically
namic conditions within the bioreactor without the use of affects the organization of cells on the substrate. A thermo-
dynamic view of the problem suggests that the overall sys-
tem (consisting of the cells and the extracellular support)
ultimately reaches an equilibrium state when the surface
free energy is minimized. According to this concept, the
existence of large cell–substrate adhesion forces relative
to cell–cell adhesion forces prevents cell–cell overlapping
(Fig. 16). In contrast, the opposite situation would lead to
cell clumping or multilayered growth on the substrate.
This prediction is in agreement with the observation of
cellular aggregate formation when hepatocytes are plated
on a nonadherent surface as opposed to a highly adherent
surface such as type I collagen.
FIGURE 15 Design of a rotating wall vessel bioreactor. Cells are
Heterotypic cell systems or “co-culture” systems have
suspended in medium, which fills the vessel until no air bubble
been used for the production of skin grafts, in long-term
is left. Oxygen is delivered via a silicone membrane in the cen-
culturesofhepatocytes,andinlong-termculturesofmixed
ter of the vessel. The vessel rotates at a relatively low speed
(∼30 rotations/min) to prevent settling of cells. bone marrow cells. These systems take advantage of the