Page 127 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 127
P1: ZBU 2nd Revised Pages
Encyclopedia of Physical Science and Technology EN002G-87 May 19, 2001 20:3
Catalyst Characterization 525
NH 3 desorption on porous glass. The addition of NH 3 had
no effect on the IR band of surface silanol groups until
enough NH 3 had been added to cover ∼1% of the surface.
Thus, the M¨ossbauer and IR results provide additional evi-
dence for the complexity of surface acid site measurement
and interpretation.
2. Positron Spectroscopy
Like M¨ossbauer spectroscopy, positron spectroscopy is
a nuclear process for probing the chemical and physical
environments of solid materials. It is a more versatile tech-
nique than M¨ossbauer spectroscopy, but it does not pro-
vide as much information on the structure surrounding the
nucleus or the immediate environment of the atom.
Applications of the technique to heterogeneous cata-
lysts have been few, but they have demonstrated that the
method is useful for catalyst characterization. For exam-
ple, the lifetime of the orthopositronium species is in-
versely proportional to the number of Br¨onsted acid sites
present in alumina–silica cracking catalysts. This interpre-
tation was derived from a correlation between the activity
for the alkylation of cumene and the lifetime of the or-
thopositronium species.
As the time resolution of the equipment improves and
computer programs become available for data reduction,
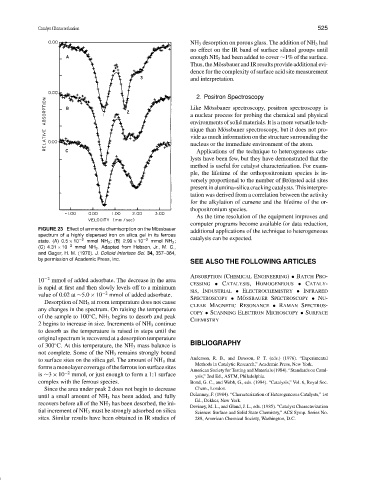
FIGURE 23 Effect of ammonia chemisorption on the M¨ossbauer
additional applications of the technique to heterogeneous
spectrum of a highly dispersed iron on silica gel in its ferrous
state. (A) 0.5 × 10 −2 mmol NH 3 ; (B) 2.99 × 10 −2 mmol NH 3 ; catalysis can be expected.
(C) 4.31 × 10 −2 mmol NH 3 . Adapted from Hobson, Jr., M. C.,
and Gager, H. M. (1970). J. Colloid Interface Sci. 34, 357–364,
by permission of Academic Press, Inc.
SEE ALSO THE FOLLOWING ARTICLES
ADSORPTION (CHEMICAL ENGINEERING) • BATCH PRO-
−2
10 mmol of added adsorbate. The decrease in the area
CESSING • CATALYSIS,HOMOGENEOUS • CATALY-
is rapid at first and then slowly levels off to a minimum SIS,INDUSTRIAL • ELECTROCHEMISTRY • INFRARED
value of 0.02 at ∼5.0 × 10 −2 mmol of added adsorbate.
SPECTROSCOPY • M ¨ OSSBAUER SPECTROSCOPY • NU-
Desorption of NH 3 at room temperature does not cause
CLEAR MAGNETIC RESONANCE • RAMAN SPECTROS-
any changes in the spectrum. On raising the temperature
COPY • SCANNING ELECTRON MICROSCOPY • SURFACE
of the sample to 100 C, NH 3 begins to desorb and peak
◦
CHEMISTRY
2 begins to increase in size. Increments of NH 3 continue
to desorb as the temperature is raised in steps until the
original spectrum is recovered at a desorption temperature
◦
of 300 C. At this temperature, the NH 3 mass balance is BIBLIOGRAPHY
not complete. Some of the NH 3 remains strongly bound
to surface sites on the silica gel. The amount of NH 3 that Anderson, R. B., and Dawson, P. T. (eds.) (1976). “Experimental
Methods in Catalytic Research,” Academic Press, New York.
formsamonolayercoverageoftheferrousionsurfacesites American Society for Testing and Materials (1984). “Standards on Catal-
is ∼3 × 10 −2 mmol, or just enough to form a 1:1 surface ysis,” 2nd Ed., ASTM, Philadelphia.
complex with the ferrous species. Bond, G. C., and Webb, G., eds. (1984). “Catalysis,” Vol. 6, Royal Soc.
Since the area under peak 2 does not begin to decrease Chem., London.
until a small amount of NH 3 has been added, and fully Delannay, F. (1984). “Characterization of Heterogeneous Catalysts,” 1st
Ed., Dekker, New York.
recovers before all of the NH 3 has been desorbed, the ini-
Deviney, M. L., and Gland, J. L., eds. (1985). ”Catalyst Characterization
tial increment of NH 3 must be strongly adsorbed on silica Science: Surface and Solid State Chemistry,” ACS Symp. Series No.
sites. Similar results have been obtained in IR studies of 288, American Chemical Society, Washington, D.C.