Page 123 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 123
P1: ZBU 2nd Revised Pages
Encyclopedia of Physical Science and Technology EN002G-87 May 19, 2001 20:3
Catalyst Characterization 521
for measuring the chemical and physical properties of a metal films onto transparent substrates, such as calcium
catalyst. The assignment of these techniques to this cate- fluoride and sodium chloride. Thin evaporated films suf-
gory has been somewhat arbitrary. Some of the techniques fer from a loss of sensitivity. Modern Fourier transform
may become routine as instrumentation and the treatment instrumentation has eliminated some of the problems of
of data improve, whereas others will provide fundamen- sensitivity and sample preparation that plagued the dis-
tal insight into the mechanisms of catalytic reactions at a persive instruments.
solid surface. All of these techniques are powerful enough The chemisorption of CO on supported metals
to provide information on the physical and chemical prop- such as platinum and palladium illustrates the tech-
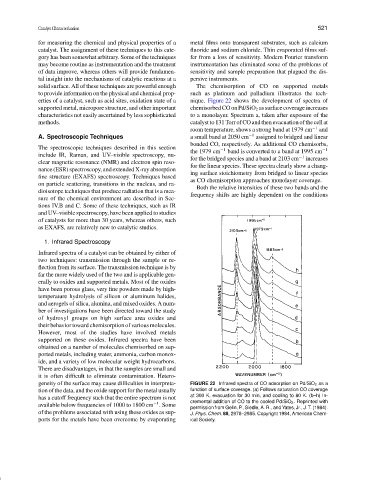
erties of a catalyst, such as acid sites, oxidation state of a nique. Figure 22 shows the development of spectra of
supported metal, micropore structure, and other important chemisorbed CO on Pd/SiO 2 as surface coverage increases
characteristics not easily ascertained by less sophisticated to a monolayer. Spectrum a, taken after exposure of the
methods. catalyst to 131 Torr of CO and then evacuation of the cell at
room temperature, shows a strong band at 1979 cm −1 and
A. Spectroscopic Techniques a small band at 2050 cm −1 assigned to bridged and linear
bonded CO, respectively. As additional CO chemisorbs,
The spectroscopic techniques described in this section −1 −1
the 1979 cm band is converted to a band at 1995 cm
include IR, Raman, and UV–visible spectroscopy, nu- −1
for the bridged species and a band at 2103 cm increases
clear magnetic resonance (NMR) and electron spin reso-
for the linear species. These spectra clearly show a chang-
nance (ESR) spectroscopy, and extended X-ray absorption
ing surface stoichiometry from bridged to linear species
fine structure (EXAFS) spectroscopy. Techniques based
as CO chemisorption approaches monolayer coverage.
on particle scattering, transitions in the nucleus, and ra-
Both the relative intensities of these two bands and the
dioisotope techniques that produce radiation that is a mea-
frequency shifts are highly dependent on the conditions
sure of the chemical environment are described in Sec-
tions IV.B and C. Some of these techniques, such as IR
and UV–visible spectroscopy, have been applied to studies
of catalysts for more than 30 years, whereas others, such
as EXAFS, are relatively new to catalytic studies.
1. Infrared Spectroscopy
Infrared spectra of a catalyst can be obtained by either of
two techniques: transmission through the sample or re-
flection from its surface. The transmission technique is by
far the more widely used of the two and is applicable gen-
erally to oxides and supported metals. Most of the oxides
have been porous glass, very fine powders made by high-
temperature hydrolysis of silicon or aluminum halides,
and aerogels of silica, alumina, and mixed oxides. A num-
ber of investigations have been directed toward the study
of hydroxyl groups on high surface area oxides and
their behavior toward chemisorption of various molecules.
However, most of the studies have involved metals
supported on these oxides. Infrared spectra have been
obtained on a number of molecules chemisorbed on sup-
ported metals, including water, ammonia, carbon monox-
ide, and a variety of low molecular weight hydrocarbons.
There are disadvantages, in that the samples are small and
it is often difficult to eliminate contamination. Hetero-
geneity of the surface may cause difficulties in interpreta- FIGURE 22 Infrared spectra of CO adsorption on Pd/SiO 2 as a
tion of the data, and the oxide support for the metal usually function of surface coverage. (a) Follows saturation CO coverage
has a cutoff frequency such that the entire spectrum is not at 300 K, evacuation for 30 min, and cooling to 80 K. (b–h) In-
cremental addition of CO to the cooled Pd/SiO 2 . Reprinted with
−1
available below frequencies of 1000 to 1800 cm . Some
permission from Gelin, P., Siedle, A. R., and Yates, Jr., J. T. (1984).
of the problems associated with using these oxides as sup- J. Phys. Chem. 88, 2978–2985. Copyright 1984, American Chem-
ports for the metals have been overcome by evaporating ical Society.