Page 118 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 118
P1: ZBU 2nd Revised Pages
Encyclopedia of Physical Science and Technology EN002G-87 May 19, 2001 20:3
516 Catalyst Characterization
generate easily read patterns; however, the subcrystals are
buried within the crystal, making them inaccessible to re-
actant molecules. Frequently the purpose of the prepara-
tion technique is to disperse the catalytic components in
such a way as to maximize their availability to reactants.
When this is done effectively, the crystal structure is com-
posed of fewer subcrystals, and thus the diffraction of X
rays is minimized since little long-range structure or few
planes exist. Thus, characterization of such materials us-
ing X rays becomes impossible. As the crystals get smaller
and smaller, the XRD peaks get broader and broader and
eventually are buried in the background; however, it is
these “X-ray-amorphous” species that are often the most
active for a given catalytic reaction.
Alternative techniques do exist, however, for obtain-
ing information regarding the distribution and number of
catalytic components dispersed within or on the support.
Selective gas adsorption, referred to as chemisorption, can
be used to measure the accessible catalytic component on
the surface indirectly by noting the amount of gas ad-
sorbed per unit weight of catalyst. The stoichiometry of
the chemisorption process must be known in order to es-
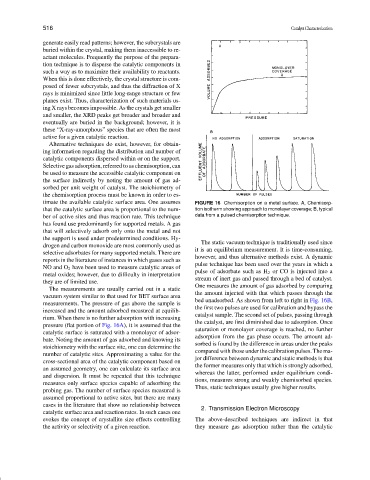
timate the available catalytic surface area. One assumes FIGURE 16 Chemisorption on a metal surface. A, Chemisorp-
that the catalytic surface area is proportional to the num- tion isotherm showing approach to monolayer coverage; B, typical
ber of active sites and thus reaction rate. This technique data from a pulsed chemisorption technique.
has found use predominantly for supported metals. A gas
that will selectively adsorb only onto the metal and not
the support is used under predetermined conditions. Hy-
The static vacuum technique is traditionally used since
drogen and carbon monoxide are most commonly used as
it is an equilibrium measurement. It is time-consuming,
selective adsorbates for many supported metals. There are
however, and thus alternative methods exist. A dynamic
reports in the literature of instances in which gases such as
pulse technique has been used over the years in which a
NO and O 2 have been used to measure catalytic areas of
pulse of adsorbate such as H 2 or CO is injected into a
metal oxides; however, due to difficulty in interpretation
stream of inert gas and passed through a bed of catalyst.
they are of limited use.
One measures the amount of gas adsorbed by comparing
The measurements are usually carried out in a static
the amount injected with that which passes through the
vacuum system similar to that used for BET surface area
bed unadsorbed. As shown from left to right in Fig. 16B,
measurements. The pressure of gas above the sample is
the first two pulses are used for calibration and bypass the
increased and the amount adsorbed measured at equilib-
catalyst sample. The second set of pulses, passing through
rium. When there is no further adsorption with increasing
the catalyst, are first diminished due to adsorption. Once
pressure (flat portion of Fig. 16A), it is assumed that the
saturation or monolayer coverage is reached, no further
catalytic surface is saturated with a monolayer of adsor-
adsorption from the gas phase occurs. The amount ad-
bate. Noting the amount of gas adsorbed and knowing its
sorbed is found by the difference in areas under the peaks
stoichiometry with the surface site, one can determine the
compared with those under the calibration pulses. The ma-
number of catalytic sites. Approximating a value for the
jor difference between dynamic and static methods is that
cross-sectional area of the catalytic component based on
the former measures only that which is strongly adsorbed,
an assumed geometry, one can calculate its surface area
whereas the latter, performed under equilibrium condi-
and dispersion. It must be repeated that this technique
tions, measures strong and weakly chemisorbed species.
measures only surface species capable of adsorbing the
Thus, static techniques usually give higher results.
probing gas. The number of surface species measured is
assumed proportional to active sites, but there are many
cases in the literature that show no relationship between
2. Transmission Electron Microscopy
catalytic surface area and reaction rates. In such cases one
evokes the concept of crystallite size effects controlling The above-described techniques are indirect in that
the activity or selectivity of a given reaction. they measure gas adsorption rather than the catalytic