Page 122 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 122
P1: ZBU 2nd Revised Pages
Encyclopedia of Physical Science and Technology EN002G-87 May 19, 2001 20:3
520 Catalyst Characterization
the active sites exist and where chemisorption, chemical
reaction, and desorption take place.
Most commonly a freshly reduced metal or metal-
supported catalyst will have a layer of oxygen, either
strongly chemisorbed or fully oxidized, on its surface. Be-
cause of this it is common practice in hydrogenation reac-
tions to pretreat the catalyst in a stream of H 2 in the reactor
to remove all traces of surface oxygen before performing
the catalytic reactions. Also, throughout the years catalytic
scientists have dealt with the problem of pyrophoric met-
als by intentionally blanketing the catalyst with a carefully
controlled amount of O 2 or CO 2 after reduction to prevent
bulk oxidation. These protective layers are removed by re-
duction and/or heat treatment in order to permit catalysis
to occur.
Platinum–rhodium alloys in the form of wires woven
into screens or gauzes are catalysts for the production of
hydrogen cyanide by the reaction of ammonia, methane,
and oxygen. The surface of these wires may contain impu-
rities from the manufacturing operations, and thus surface
analysis as opposed to bulk analysis is more important for
predicting reaction rates. During ammonia oxidation, the
platinum–rhodium alloy becomes surface enriched with
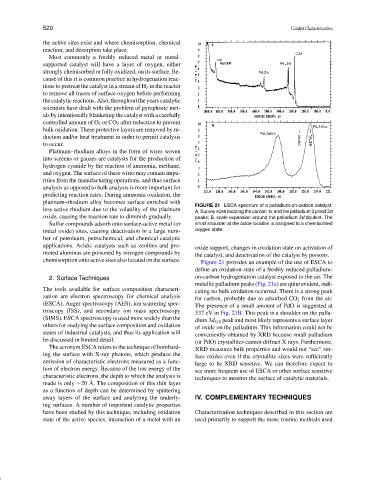
FIGURE 21 ESCA spectrum of a palladium-on-carbon catalyst.
less active rhodium due to the volatility of the platinum
A, Survey scan locating the carbon 1s and the palladium 3pand 3d
oxide, causing the reaction rate to diminish gradually. peaks; B, scale expansion around the palladium 3d doublet. The
Sulfur compounds adsorb onto surface-active metal (or small shoulder at the oxide location is assigned to a chemisorbed
metal oxide) sites, causing deactivation in a large num- oxygen state.
ber of petroleum, petrochemical, and chemical catalytic
applications. Acidic catalysts such as zeolites and pro-
oxide support, changes in oxidation state on activation of
moted aluminas are poisoned by nitrogen compounds by
the catalyst, and deactivation of the catalyst by poisons.
chemisorption onto active sites also located on the surface.
Figure 21 provides an example of the use of ESCA to
define an oxidation state of a freshly reduced palladium-
2. Surface Techniques on-carbon hydrogenation catalyst exposed to the air. The
metallic palladium peaks (Fig. 21a) are quite evident, indi-
The tools available for surface composition characteri-
cating no bulk oxidation occurred. There is a strong peak
zation are electron spectroscopy for chemical analysis
for carbon, probably due to adsorbed CO 2 from the air.
(ESCA), Auger spectroscopy (AES), ion scattering spec- The presence of a small amount of PdO is suggested at
troscopy (ISS), and secondary ion mass spectroscopy 337 eV in Fig. 21B. This peak is a shoulder on the palla-
(SIMS). ESCA spectroscopy is used more widely than the dium 3d 5/2 peak and most likely represents a surface layer
others for studying the surface composition and oxidation of oxide on the palladium. This information could not be
states of industrial catalysts, and thus its application will conveniently obtained by XRD because small palladium
be discussed in limited detail. (or PdO) crystallites cannot diffract X rays. Furthermore,
The acronym ESCA refers to the technique of bombard- XRD measures bulk properties and would not “see” sur-
ing the surface with X-ray photons, which produce the face oxides even if the crystallite sizes were sufficiently
emission of characteristic electrons measured as a func- large to be XRD sensitive. We can therefore expect to
tion of electron energy. Because of the low energy of the
see more frequent use of ESCA or other surface sensitive
characteristic electrons, the depth to which the analysis is techniques to monitor the surface of catalytic materials.
˚
made is only ∼20 A. The composition of this thin layer
as a function of depth can be determined by sputtering
away layers of the surface and analyzing the underly- IV. COMPLEMENTARY TECHNIQUES
ing surfaces. A number of important catalytic properties
have been studied by this technique, including oxidation Characterization techniques described in this section are
state of the active species, interaction of a metal with an used primarily to support the more routine methods used