Page 117 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 117
P1: ZBU 2nd Revised Pages
Encyclopedia of Physical Science and Technology EN002G-87 May 19, 2001 20:3
Catalyst Characterization 515
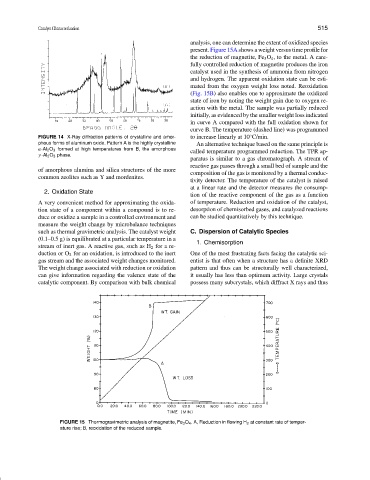
analysis, one can determine the extent of oxidized species
present. Figure 15A shows a weight versus time profile for
the reduction of magnetite, Fe 3 O 4 , to the metal. A care-
fully controlled reduction of magnetite produces the iron
catalyst used in the synthesis of ammonia from nitrogen
and hydrogen. The apparent oxidation state can be esti-
mated from the oxygen weight loss noted. Reoxidation
(Fig. 15B) also enables one to approximate the oxidized
state of iron by noting the weight gain due to oxygen re-
action with the metal. The sample was partially reduced
initially, as evidenced by the smaller weight loss indicated
in curve A compared with the full oxidation shown for
curve B. The temperature (dashed line) was programmed
◦
FIGURE 14 X-Ray diffraction patterns of crystalline and amor- to increase linearly at 10 C/min.
phous forms of aluminum oxide. Pattern A is the highly crystalline An alternative technique based on the same principle is
α-Al 2 O 3 formed at high temperatures from B, the amorphous
called temperature programmed reduction. The TPR ap-
γ -Al 2 O 3 phase.
paratus is similar to a gas chromatograph. A stream of
reactive gas passes through a small bed of sample and the
of amorphous alumina and silica structures of the more
composition of the gas is monitored by a thermal conduc-
common zeolites such as Y and mordenites.
tivity detector. The temperature of the catalyst is raised
at a linear rate and the detector measures the consump-
2. Oxidation State
tion of the reactive component of the gas as a function
A very convenient method for approximating the oxida- of temperature. Reduction and oxidation of the catalyst,
tion state of a component within a compound is to re- desorption of chemisorbed gases, and catalyzed reactions
duce or oxidize a sample in a controlled environment and can be studied quantitatively by this technique.
measure the weight change by microbalance techniques
such as thermal gravimetric analysis. The catalyst weight C. Dispersion of Catalytic Species
(0.1–0.5 g) is equilibrated at a particular temperature in a
1. Chemisorption
stream of inert gas. A reactive gas, such as H 2 for a re-
duction or O 2 for an oxidation, is introduced to the inert One of the most frustrating facts facing the catalytic sci-
gas stream and the associated weight changes monitored. entist is that often when a structure has a definite XRD
The weight change associated with reduction or oxidation pattern and thus can be structurally well characterized,
can give information regarding the valence state of the it usually has less than optimum activity. Large crystals
catalytic component. By comparison with bulk chemical possess many subcrystals, which diffract X rays and thus
FIGURE 15 Thermogravimetric analysis of magnetite, Fe 3 O 4 . A, Reduction in flowing H 2 at constant rate of temper-
ature rise; B, reoxidation of the reduced sample.