Page 115 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 115
P1: ZBU 2nd Revised Pages
Encyclopedia of Physical Science and Technology EN002G-87 May 19, 2001 20:3
Catalyst Characterization 513
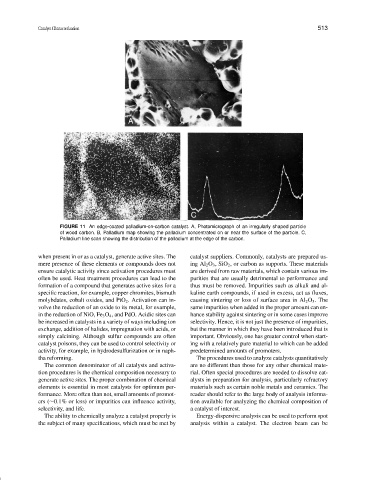
FIGURE 11 An edge-coated palladium-on-carbon catalyst. A, Photomicrograph of an irregularly shaped particle
of wood carbon. B, Palladium map showing the palladium concentrated on or near the surface of the particle. C,
Palladium line scan showing the distribution of the palladium at the edge of the carbon.
when present in or as a catalyst, generate active sites. The catalyst suppliers. Commonly, catalysts are prepared us-
mere presence of these elements or compounds does not ing Al 2 O 3 , SiO 2 , or carbon as supports. These materials
ensure catalytic activity since activation procedures must are derived from raw materials, which contain various im-
often be used. Heat treatment procedures can lead to the purities that are usually detrimental to performance and
formation of a compound that generates active sites for a thus must be removed. Impurities such as alkali and al-
specific reaction, for example, copper chromites, bismuth kaline earth compounds, if used in excess, act as fluxes,
molybdates, cobalt oxides, and PtO 2 . Activation can in- causing sintering or loss of surface area in Al 2 O 3 . The
volve the reduction of an oxide to its metal, for example, same impurities when added in the proper amount can en-
in the reduction of NiO, Fe 3 O 4 , and PdO. Acidic sites can hance stability against sintering or in some cases improve
be increased in catalysts in a variety of ways including ion selectivity. Hence, it is not just the presence of impurities,
exchange, addition of halides, impregnation with acids, or but the manner in which they have been introduced that is
simply calcining. Although sulfur compounds are often important. Obviously, one has greater control when start-
catalyst poisons, they can be used to control selectivity or ing with a relatively pure material to which can be added
activity, for example, in hydrodesulfurization or in naph- predetermined amounts of promoters.
tha reforming. The procedures used to analyze catalysts quantitatively
The common denominator of all catalysts and activa- are no different than those for any other chemical mate-
tion procedures is the chemical composition necessary to rial. Often special procedures are needed to dissolve cat-
generate active sites. The proper combination of chemical alysts in preparation for analysis, particularly refractory
elements is essential in most catalysts for optimum per- materials such as certain noble metals and ceramics. The
formance. More often than not, small amounts of promot- reader should refer to the large body of analysis informa-
ers (∼0.1% or less) or impurities can influence activity, tion available for analyzing the chemical composition of
selectivity, and life. a catalyst of interest.
The ability to chemically analyze a catalyst properly is Energy-dispersive analysis can be used to perform spot
the subject of many specifications, which must be met by analysis within a catalyst. The electron beam can be