Page 116 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 116
P1: ZBU 2nd Revised Pages
Encyclopedia of Physical Science and Technology EN002G-87 May 19, 2001 20:3
514 Catalyst Characterization
focused on a particle or area whose analysis is desired,
and the X rays characteristic of the elements present are
measured. This is a common method for poison analysis in
a selected area of the catalyst. It should not be a substitute
for a total analysis since only a small area is analyzed.
2. Analysis by X-Ray Diffraction
Provided that a material is sufficiently crystalline to
diffract X rays and is present in an amount greater
than ∼1%, X-ray diffraction (XRD) can be used for
qualitative and quantitative analyses. The principle of this
technique is that crystal structures possess planes made
by repetitive arrangements of atoms, which are capable FIGURE 13 X-Ray diffraction patterns of a standard NaY zeolite
(A) and a typical cracking catalyst containing the zeolite (B).
of diffracting X rays. The angles of diffraction differ for
the various planes within the crystal, and thus every com-
pound or element has its own somewhat unique diffraction
zeolite present is important for quality control as well as
pattern. The differences in these patterns, therefore, allow
for defining the temperature limits of crystalline structure
the differentiation of various structures within the
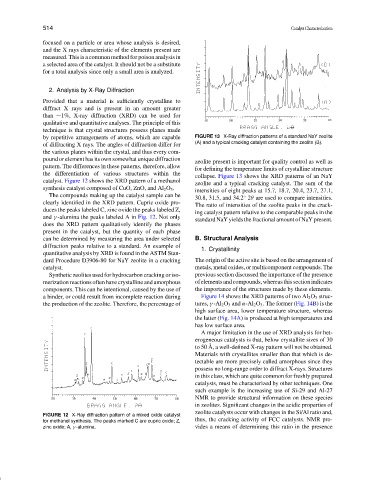
collapse. Figure 13 shows the XRD patterns of an NaY
catalyst. Figure 12 shows the XRD pattern of a methanol
zeolite and a typical cracking catalyst. The sum of the
synthesis catalyst composed of CuO, ZnO, and Al 2 O 3 .
intensities of eight peaks at 15.7, 18.7, 20.4, 23.7, 27.1,
The compounds making up the catalyst sample can be
30.8, 31.5, and 34.2 2θ are used to compare intensities.
◦
clearly identified in the XRD pattern. Cupric oxide pro-
The ratio of intensities of the zeolite peaks in the crack-
duces the peaks labeled C, zinc oxide the peaks labeled Z,
ing catalyst pattern relative to the comparable peaks in the
and γ -alumina the peaks labeled A in Fig. 12. Not only
standard NaY yields the fractional amount of NaY present.
does the XRD pattern qualitatively identify the phases
present in the catalyst, but the quantity of each phase
can be determined by measuring the area under selected B. Structural Analysis
diffraction peaks relative to a standard. An example of
1. Crystallinity
quantitative analysis by XRD is found in the ASTM Stan-
dard Procedure D3906-80 for NaY zeolite in a cracking The origin of the active site is based on the arrangement of
catalyst. metals, metal oxides, or multicomponent compounds. The
Synthetic zeolites used for hydrocarbon cracking or iso- previous section discussed the importance of the presence
merization reactions often have crystalline and amorphous ofelementsandcompounds,whereasthissectionindicates
components. This can be intentional, caused by the use of the importance of the structures made by these elements.
a binder, or could result from incomplete reaction during Figure 14 shows the XRD patterns of two Al 2 O 3 struc-
the production of the zeolite. Therefore, the percentage of tures, γ -Al 2 O 3 and α-Al 2 O 3 . The former (Fig. 14B) is the
high surface area, lower temperature structure, whereas
the latter (Fig. 14A) is produced at high temperatures and
has low surface area.
A major limitation in the use of XRD analysis for het-
erogeneous catalysts is that, below crystallite sizes of 30
˚
to 50 A, a well-defined X-ray pattern will not be obtained.
Materials with crystallites smaller than that which is de-
tectable are more precisely called amorphous since they
possess no long-range order to diffract X-rays. Structures
in this class, which are quite common for freshly prepared
catalysts, must be characterized by other techniques. One
such example is the increasing use of Si-29 and Al-27
NMR to provide structural information on these species
in zeolites. Significant changes in the acidic properties of
zeolite catalysts occur with changes in the Si/Al ratio and,
FIGURE 12 X-Ray diffraction pattern of a mixed oxide catalyst
for methanol synthesis. The peaks marked C are cupric oxide; Z, thus, the cracking activity of FCC catalysts. NMR pro-
zinc oxide; A, γ -alumina. vides a means of determining this ratio in the presence