Page 32 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 32
P1: FJD Revised Pages
Encyclopedia of Physical Science and Technology EN001-13 May 7, 2001 12:29
254 Adsorption (Chemical Engineering)
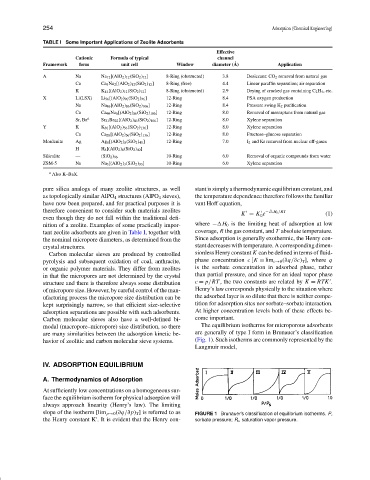
TABLE I Some Important Applications of Zeolite Adsorbents
Effective
Cationic Formula of typical channel
Framework form unit cell Window diameter ( ˚ A) Application
A Na Na 12 [(AlO 2 ) 12 (SiO 2 ) 12 ] 8-Ring (obstructed) 3.8 Desiccant: CO 2 removal from natural gas
Ca Ca 5 Na 2 [(AlO 2 ) 12 (SiO 2 ) 12 ] 8-Ring (free) 4.4 Linear paraffin separation; air separation
K K 12 [(AlO 2 ) 12 (SiO 2 ) 12 ] 8-Ring (obstructed) 2.9 Drying of cracked gas containing C 2 H 4 , etc.
X Li(LSX) Li 96 [(AlO 2 ) 96 (SiO 2 ) 96 ] 12-Ring 8.4 PSA oxygen production
Na Na 86 [(AlO 2 ) 86 (SiO 2 ) 106 ] 12-Ring 8.4 Pressure swing H 2 purification
Ca Ca 40 Na 6 [(AlO 2 ) 86 (SiO 2 ) 106 ] 12-Ring 8.0 Removal of mercaptans from natural gas
Sr, Ba a Sr 21 Ba 22 [(AlO 2 ) 86 (SiO 2 ) 106 ] 12-Ring 8.0 Xylene separation
Y K K 56 [(AlO 2 ) 56 (SiO 2 ) 136 ] 12-Ring 8.0 Xylene separation
Ca Ca 28 [(AlO 2 ) 56 (SiO 2 ) 136 ] 12-Ring 8.0 Fructose–glucose separation
Mordenite Ag Ag 8 [(AlO 2 ) 8 (SiO 2 ) 40 ] 12-Ring 7.0 I 2 and Kr removal from nuclear off-gases
H H 8 [(AlO 2 ) 8 (SiO 2 ) 40 ]
Silicalite — (SiO 2 ) 96 10-Ring 6.0 Removal of organic compounds from water
ZSM-5 Na Na 3 [(AlO 2 ) 3 (SiO 2 ) 93 ] 10-Ring 6.0 Xylene separation
a Also K–BaX.
pure silica analogs of many zeolite structures, as well stant is simply a thermodynamic equilibrium constant, and
as topologically similar AlPO 4 structures (AlPO 4 sieves), the temperature dependence therefore follows the familiar
have now been prepared, and for practical purposes it is vant Hoff equation,
therefore convenient to consider such materials zeolites − H 0 /RT
K = K e (1)
0
even though they do not fall within the traditional defi-
nition of a zeolite. Examples of some practically impor- where − H 0 is the limiting heat of adsorption at low
tant zeolite adsorbents are given in Table I, together with coverage, R the gas constant, and T absolute temperature.
the nominal micropore diameters, as determined from the Since adsorption is generally exothermic, the Henry con-
crystal structures. stant decreases with temperature. A corresponding dimen-
Carbon molecular sieves are produced by controlled sionless Henry constant K can be defined in terms of fluid-
pyrolysis and subsequent oxidation of coal, anthracite, phase concentration c [K = lim c→0 (∂q/∂c) T ], where q
or organic polymer materials. They differ from zeolites is the sorbate concentration in adsorbed phase, rather
in that the micropores are not determined by the crystal than partial pressure, and since for an ideal vapor phase
structure and there is therefore always some distribution c = p/RT , the two constants are related by K = RTK .
of micropore size. However, by careful control of the man- Henry’s law corresponds physically to the situation where
ufacturing process the micropore size distribution can be the adsorbed layer is so dilute that there is neither compe-
kept surprisingly narrow, so that efficient size-selective tition for adsorption sites nor sorbate–sorbate interaction.
adsorption separations are possible with such adsorbents. At higher concentration levels both of these effects be-
Carbon molecular sieves also have a well-defined bi- come important.
modal (macropore–micropore) size distribution, so there The equilibrium isotherms for microporous adsorbents
are many similarities between the adsorption kinetic be- are generally of type I form in Brunauer’s classification
havior of zeolitic and carbon molecular sieve systems. (Fig. 1). Such isotherms are commonly represented by the
Langmuir model,
IV. ADSORPTION EQUILIBRIUM
A. Thermodynamics of Adsorption
At sufficiently low concentrations on a homogeneous sur-
face the equilibrium isotherm for physical adsorption will
always approach linearity (Henry’s law). The limiting
slope of the isotherm [lim p→0 (∂q/∂p) T ] is referred to as FIGURE 1 Brunauer’s classification of equilibrium isotherms. P,
the Henry constant K . It is evident that the Henry con- sorbate pressure; P s , saturation vapor pressure.