Page 33 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 33
P1: FJD Revised Pages
Encyclopedia of Physical Science and Technology EN001-13 May 7, 2001 12:29
Adsorption (Chemical Engineering) 255
q/q s = bp/(1 + bp) (2) When the equilibrium constant b is large (highly favor-
able adsorption) the Langmuir isotherm approaches irre-
where q s is the saturation capacity and b an equilibrium
versible or rectangular form,
constant that is directly related to the Henry constant ∗ ∗
(K = bq s ). To a first approximation q s is independent of p = 0, q = 0; p > 0, q = q s (3)
temperature, so the temperature dependence of b is the where q represents the equilibrium constant ratio in the
∗
same as that of the Henry constant [Eq. (1)]. The Langmuir adsorbed phase. This provides the basis for a very use-
model was originally derived for localized chemisorption ful limiting case, which is widely used in the analysis of
on an ideal surface with no interaction between adsorbed adsorption column dynamics since the solutions for a rect-
molecules, but with certain approximations the same form angular isotherm are generally relatively simple and they
of equation can be derived for mobile physical adsorption provide a reasonably reliable prediction of the behaviour
at moderate coverage. Although this model provides a that can be expected for a real system when the isotherm
quantitatively accurate description of the isotherms for is highly favorable.
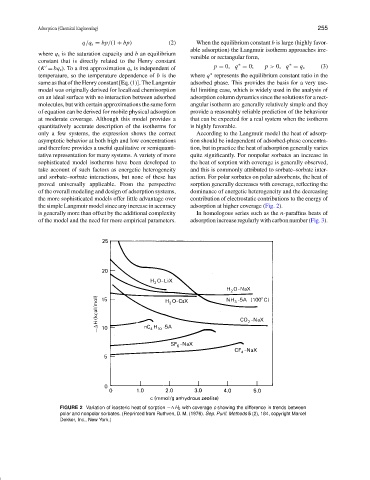
only a few systems, the expression shows the correct According to the Langmuir model the heat of adsorp-
asymptotic behavior at both high and low concentrations tion should be independent of adsorbed-phase concentra-
and therefore provides a useful qualitative or semiquanti- tion, but in practice the heat of adsorption generally varies
tative representation for many systems. A variety of more quite significantly. For nonpolar sorbates an increase in
sophisticated model isotherms have been developed to the heat of sorption with coverage is generally observed,
take account of such factors as energetic heterogeneity and this is commonly attributed to sorbate–sorbate inter-
and sorbate–sorbate interactions, but none of these has action. For polar sorbates on polar adsorbents, the heat of
proved universally applicable. From the perspective sorption generally decreases with coverage, reflecting the
of the overall modeling and design of adsorption systems, dominance of energetic heterogeneity and the decreasing
the more sophisticated models offer little advantage over contribution of electrostatic contributions to the energy of
the simple Langmuir model since any increase in accuracy adsorption at higher coverage (Fig. 2).
is generally more than offset by the additional complexity In homologous series such as the n-paraffins heats of
of the model and the need for more empirical parameters. adsorption increase regularly with carbon number (Fig. 3).
FIGURE 2 Variation of isosteric heat of sorption − H 0 with coverage c showing the difference in trends between
polar and nonpolar sorbates. (Reprinted from Ruthven, D. M. (1976). Sep. Purif. Methods 5 (2), 184, copyright Marcel
Dekker, Inc., New York.)