Page 36 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 36
P1: FJD Revised Pages
Encyclopedia of Physical Science and Technology EN001-13 May 7, 2001 12:29
258 Adsorption (Chemical Engineering)
B. Macropore Diffusion
Diffusion in macropores occurs mainly by the combined
effects of bulk molecular diffusion (as in the free fluid) and
Knudsen flow, with generally smaller contributions from
other mechanisms such as surface diffusion and Poiseuille
flow. Knudsen flow, which has the characteristics of a
diffusive process, occurs because molecules striking the
pore wall are instantaneously adsorbed and re-emitted in
a random direction. The relative importance of bulk and
Knudsen diffusion depends on the relative frequency of
molecule–molecule and molecule–wall collisions, which
in turn depends on the ratio of the mean free path to pore
diameter. Thus Knudsen flow becomes dominant in small
pores at low pressures, while in larger pores and at higher
pressures diffusion occurs mainly by the molecular mech-
anism. Since the mechanism of diffusion may well be dif-
ferent at different pressures, one must be cautious about
extrapolating from experimental diffusivity data, obtained
at low pressures, to the high pressures commonly em-
ployed in industrial processes.
The combined effects of Knudsen, D K , and molecular
(fluid-phase) diffusion D m are commonly estimated from
the expression:
1 1 1
= τ + (12)
D p D m D K
where τ is an empirical factor, characteristic of the ad-
sorbent, that corrects for the effects of pore direction and
nonuniform pore diameter. Modeling the pore structure
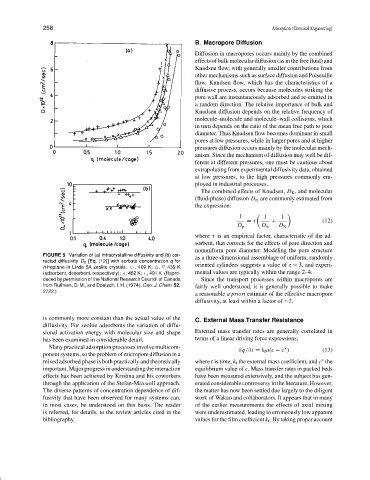
FIGURE 5 Variation of (a) intracrystalline diffusivity and (b) cor-
as a three-dimensional assemblage of uniform, randomly
rected diffusivity D 0 [Eq. (12)] with sorbate concentration q for
oriented cylinders suggests a value of τ = 3, and experi-
n-heptane in Linde 5A zeolite crystals. ❤ , 409 K; , 439 K
(adsorbent, desorbent, respectively); ×, 462 K; +, 491 K. (Repro- mental values are typically within the range 2–4.
duced by permission of the National Research Council of Canada Since the transport processes within macropores are
from Ruthven, D. M., and Doetsch, I. H. (1974). Can. J. Chem. 52, fairly well understood, it is generally possible to make
2722.)
a reasonable a priori estimate of the effective macropore
diffusivity, at least within a factor of ∼2.
is commonly more constant than the actual value of the C. External Mass Transfer Resistance
diffusivity. For zeolite adsorbents the variation of diffu-
sional activation energy with molecular size and shape External mass transfer rates are generally correlated in
has been examined in considerable detail. terms of a linear driving force expressions,
Many practical adsorption processes involve multicom- ∗
∂q/∂t = k f a(c − c ) (13)
ponent systems, so the problem of micropore diffusion in a
∗
mixed adsorbed phase is both practically and theoretically where t is time, k f the external mass coefficient, and c the
important.Majorprogressinunderstandingtheinteraction equilibrium value of c. Mass transfer rates in packed beds
effects has been achieved by Krishna and his coworkers have been measured extensively, and the subject has gen-
through the application of the Stefan-Maxwell approach. erated considerable controversy in the literature. However,
The diverse patterns of concentration dependence of dif- the matter has now been settled due largely to the diligent
fusivity that have been observed for many systems can, work of Wakao and collaborators. It appears that in many
in most cases, be understood on this basis. The reader of the earlier measurements the effects of axial mixing
is referred, for details, to the review articles cited in the were underestimated, leading to erroneously low apparent
bibliography. values for the film coefficient k f . By taking proper account