Page 320 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 320
P1: GSY/GSR/GLT P2: GLM Final Pages
Encyclopedia of Physical Science and Technology EN007E-968 June 30, 2001 17:35
366 High-Pressure Synthesis (Chemistry)
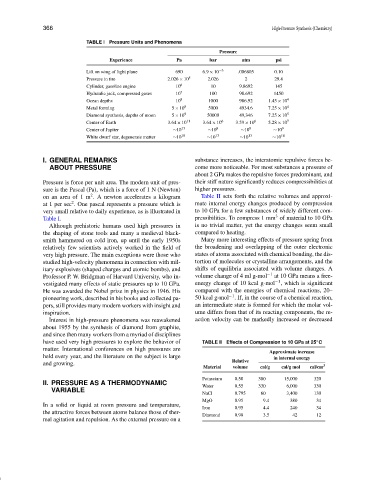
TABLE I Pressure Units and Phenomena
Pressure
Experience Pa bar atm psi
Lift on wing of light plane 690 6.9 × 10 −3 .006805 0.10
Pressure in tire 2.026 × 10 5 2.026 2 29.4
Cylinder, gasoline engine 10 6 10 9.8692 145
Hydraulic jack, compressed gases 10 7 100 98.692 1450
Ocean depths 10 8 1000 986.92 1.45 × 10 4
Metal forming 5 × 10 8 5000 4934.6 7.25 × 10 4
Diamond synthesis, depths of moon 5 × 10 9 50000 49,346 7.25 × 10 5
Center of Earth 3.64 × 10 11 3.64 × 10 6 3.59 × 10 6 5.28 × 10 7
Center of Jupiter ∼10 13 ∼10 8 ∼10 8 ∼10 9
White dwarf star, degenerate matter ∼10 18 ∼10 13 ∼10 13 ∼10 14
I. GENERAL REMARKS substance increases, the interatomic repulsive forces be-
ABOUT PRESSURE come more noticeable. For most substances a pressure of
about 2 GPa makes the repulsive forces predominant, and
Pressure is force per unit area. The modern unit of pres- their stiff nature significantly reduces compressibilities at
sure is the Pascal (Pa), which is a force of 1 N (Newton) higher pressures.
2
on an area of 1 m . A newton accelerates a kilogram Table II sets forth the relative volumes and approxi-
2
at 1 per sec . One pascal represents a pressure which is mate internal energy changes produced by compression
very small relative to daily experience, as is illustrated in to 10 GPa for a few substances of widely different com-
3
Table I. pressibilities. To compress 1 mm of material to 10 GPa
Although prehistoric humans used high pressures in is no trivial matter, yet the energy changes seem small
the shaping of stone tools and many a medieval black- compared to heating.
smith hammered on cold iron, up until the early 1950s Many more interesting effects of pressure spring from
relatively few scientists actively worked in the field of the broadening and overlapping of the outer electronic
very high pressure. The main exceptions were those who states of atoms associated with chemical bonding, the dis-
studied high-velocity phenomena in connection with mil- tortion of molecules or crystalline arrangements, and the
itary explosives (shaped charges and atomic bombs), and shifts of equilibria associated with volume changes. A
Professor P. W. Bridgman of Harvard University, who in- volume change of 4 ml g-mol −1 at 10 GPa means a free-
−1
vestigated many effects of static pressures up to 10 GPa. energy change of 10 kcal g-mol , which is significant
He was awarded the Nobel prize in physics in 1946. His compared with the energies of chemical reactions, 20–
−1
pioneering work, described in his books and collected pa- 50 kcal g-mol . If, in the course of a chemical reaction,
pers, still provides many modern workers with insight and an intermediate state is formed for which the molar vol-
inspiration. ume differs from that of its reacting components, the re-
Interest in high-pressure phenomena was reawakened action velocity can be markedly increased or decreased
about 1955 by the synthesis of diamond from graphite,
and since then many workers from a myriad of disciplines
have used very high pressures to explore the behavior of TABLE II Effects of Compression to 10 GPa at 25 C
◦
matter. International conferences on high pressures are
Approximate increase
held every year, and the literature on the subject is large in internal energy
Relative
and growing.
Material volume cal/g cal/g mol cal/cm 3
Potassium 0.50 380 15,000 320
II. PRESSURE AS A THERMODYNAMIC
Water 0.55 330 6,000 330
VARIABLE
NaCl 0.795 60 3,400 130
MgO 0.95 9.4 380 34
In a solid or liquid at room pressure and temperature,
Iron 0.95 4.4 240 34
the attractive forces between atoms balance those of ther-
Diamond 0.98 3.5 42 12
mal agitation and repulsion. As the external pressure on a