Page 339 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 339
P1: GRB/MBQ P2: GNH Final Pages
Encyclopedia of Physical Science and Technology EN009H-407 July 18, 2001 23:34
176 Mass Transfer and Diffusion
coefficient of hydrogen sulfide to be different than the
diffusion coefficient of hydrogen, because these are two
different chemical species. However, the dispersion coef-
ficient of hydrogen sulfide in the smoke will be the same
as the dispersion coefficient of the hydrogen in the smoke
because the mechanism is not that of molecular motion,
but rather of velocity fluctuations.
Dispersion coefficients are usually much greater than
diffusion coefficients and cause much more rapid mixing
than would ever be possible from molecular motion alone
(Cussler, 1997). In particular, for turbulent flow in a pipe,
the dispersion coefficient is given by
E = dv/2 (28)
where d is the pipe diameter and v is the average velocity
of the fluid in the pipe. However, if the flow in the pipe is
laminar instead of turbulent, the corresponding result is
2 2
d v
E = (29)
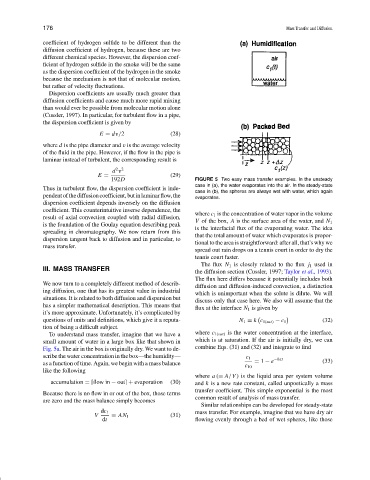
192D FIGURE 5 Two easy mass transfer examples. In the unsteady
case in (a), the water evaporates into the air. In the steady-state
Thus in turbulent flow, the dispersion coefficient is inde-
case in (b), the spheres are always wet with water, which again
pendentofthediffusioncoefficient,butinlaminarflow,the evaporates.
dispersion coefficient depends inversely on the diffusion
coefficient. This counterintuitive inverse dependence, the
where c 1 is the concentration of water vapor in the volume
result of axial convection coupled with radial diffusion,
V of the box, A is the surface area of the water, and N 1
is the foundation of the Goulay equation describing peak
is the interfacial flux of the evaporating water. The idea
spreading in chromatography. We now return from this
that the total amount of water which evaporates is propor-
dispersion tangent back to diffusion and in particular, to
tional to the area is straightforward: after all, that’swhywe
mass transfer.
spread out rain drops on a tennis court in order to dry the
tennis court faster.
The flux N 1 is closely related to the flux j 1 used in
III. MASS TRANSFER
the diffusion section (Cussler, 1997; Taylor et al., 1993).
The flux here differs because it potentially includes both
We now turn to a completely different method of describ-
diffusion and diffusion-induced convection, a distinction
ing diffusion, one that has its greatest value in industrial
which is unimportant when the solute is dilute. We will
situations. It is related to both diffusion and dispersion but
discuss only that case here. We also will assume that the
has a simpler mathematical description. This means that
flux at the interface N 1 is given by
it’s more approximate. Unfortunately, it’s complicated by
questions of units and definitions, which give it a reputa- N 1 = k c 1(sat) − c 1 (32)
tion of being a difficult subject.
To understand mass transfer, imagine that we have a where c 1(sat) is the water concentration at the interface,
small amount of water in a large box like that shown in which is at saturation. If the air is initially dry, we can
Fig. 5a. The air in the box is originally dry. We want to de- combine Eqs. (31) and (32) and integrate to find
scribe the water concentration in the box—the humidity— c 1 −kat
as a function of time. Again, we begin with a mass balance = 1 − e (33)
c 10
like the following
where a (= A/V ) is the liquid area per system volume
accumulation = [flow in − out] + evaporation (30) and k is a new rate constant, called unpoetically a mass
transfer coefficient. This simple exponential is the most
Because there is no flow in or out of the box, those terms
common result of analysis of mass transfer.
are zero and the mass balance simply becomes
Similar relationships can be developed for steady-state
dc 1 mass transfer. For example, imagine that we have dry air
V = AN 1 (31)
dt flowing evenly through a bed of wet spheres, like those