Page 100 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 100
P1: LLL/LLL P2: FJU Final Pages
Encyclopedia of Physical Science and Technology EN002C-80 May 25, 2001 20:18
408 Carbohydrates
termed heteroglycans. Each of these can be either linear
or branched.
1. Linear Polysaccharides
Linear polysaccharides are formed when the hemiacetal
hydroxyl group (on C-1 of an aldose) is replaced (substi-
tuted) by a hydroxyl group from an adjoining monosac-
charideunit.Whenthisisrepeated,alinearchainisformed
that has at one end (called the reducing end) a reducing
monosaccharide (one that has a free C-1 hydroxyl group)
and at the other end (referred to as the nonreducing end)
a glycosidically linked saccharide.
The glycosidic linkage in polysaccharides is usually re-
peated in a regular manner, with little or no randomness.
However, even such seemingly regular molecules as cel-
lulose and amylose have irregularities in their structures.
Rare as these may be (sometimes the frequency is only
one in a thousand), these irregularities are real. They are
attributable to the fact that within the enzyme–substrate
system there do occur during the course of time changes,
either through abnormal action on the part of the princi-
pal chainsynthesizing enzyme or through interference of
a second enzyme. The most abundant linear polysaccha-
ride is cellulose, a glucan linked by β-D-(1 → 4) bonds,
which is present in greater quantity than all other polysac-
charides combined. Another important linear polysaccha-
ride is amylose, which is a glucan linked by α-D-(1 → 4)
linkages.
2. Branched Polysaccharides
When the hemiacetal hydroxyl groups of two monosac-
charides are replaced by two hydroxyl groups belonging
to a third sugar residue, a branching point is produced in
the molecule, which then becomes a branched polysac-
charide. The molecule may contain a single or numerous
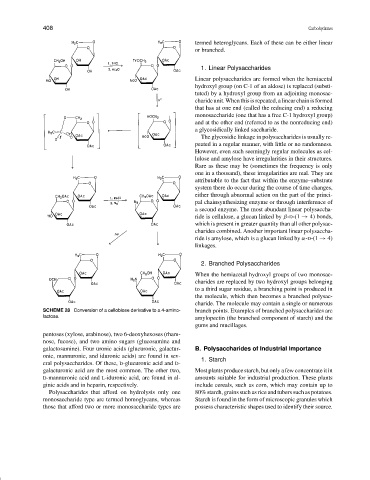
SCHEME 28 Conversion of a cellobiose derivative to a 4-amino- branch points. Examples of branched polysaccharides are
lactose.
amylopectin (the branched component of starch) and the
gums and mucillages.
pentoses (xylose, arabinose), two 6-deoxyhexoses (rham-
nose, fucose), and two amino sugars (glucosamine and
galactosamine). Four uronic acids (glucuronic, galactur- B. Polysaccharides of Industrial Importance
onic, mannuronic, and iduronic acids) are found in sev-
1. Starch
eral polysaccharides. Of these, D-glucuronic acid and D-
galacturonic acid are the most common. The other two, Most plants produce starch, but only a few concentrate it in
D-mannuronic acid and L-iduronic acid, are found in al- amounts suitable for industrial production. These plants
ginic acids and in heparin, respectively. include cereals, such as corn, which may contain up to
Polysaccharides that afford on hydrolysis only one 80% starch, grains such as rice and tubers such as potatoes.
monosaccharide type are termed homoglycans, whereas Starch is found in the form of microscopic granules which
those that afford two or more monosaccharide types are possess characteristic shapes used to identify their source.