Page 95 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 95
P1: LLL/LLL P2: FJU Final Pages
Encyclopedia of Physical Science and Technology EN002C-80 May 25, 2001 20:18
Carbohydrates 403
case, the glycosyl halide is subjected to a Koenigs–Knorr benzoyl group) is attached to O-2 of the molecule un-
type of reaction, with a halogen acceptor (e.g., AgCO 3 ) dergoing nucleophilic attack, then a Koenigs–Knorr or
as catalyst, whereas when an ester is used, a Helfrich Helfrich type of reaction will afford a glycoside hav-
type of reaction is carried out with a Lewis acid cata- ing a trans-1.2 configuration. This isomer is favored be-
lyst, such as SbCl 6 ,BF 3 , SnCl 4 ,TiF 4 , etc. The glyco- cause the intermediate cyclic carbonium ion is attacked
syl halides are best prepared from peracetylated or per- from the side opposing the ring. This is why β iso-
benzoylated mono- or disaccharides by treatment with mers are obtained from D-glucopyranosyl halides (and D-
dichloromethyl methylether (DCMME). Other good leav- galactopyranosyl halides) and α isomers are formed from
ing groups are thioglycosides, which requires a promoter D-mannopyranosyl halides.
such as dimethyl(methylthio)sulfonium triflate (DMTST)
and trichloroacetimidate which requires no activator and To obtain a cis-1,2 configuration (e.g., a glycoside hav-
is therefore often used in oligosaccharide syntheses on ing an α-D-glucopyranosyl, an α-D-galactopyranosyl, or
fixed bed polymers, discussed below. The anomeric con- a β-D-mannopyranosyl configuration), the OH-2 of the
figuration of the formed glycosidic bond is controlled by sugar moiety undergoing nucleophillic attack must be pro-
the nature of the protecting group adjacent to the leaving tected by a nonparticipating group, such as a benzyl group.
group. Participating groups such the acetyl group lead to In this case, a mixture of α and β isomers is obtained,
1,2 trans glycosidic linkages and nonparticipating groups which can be separated by chromatography. The compo-
such as the benzyl group form 1,2-cis glycosidic linkages. sition of this mixture is influenced by anomeric effects,
In both cases the leaving group and the protecting group which favor α-D anomers, and by temperature and dura-
must be removed under mild conditions that do not af- tion of reaction time, which favor the thermodynamically
fect the glycosidic linkages (bases in the first type and more stable product (i.e., the one with equatorial substi-
hydrogenation in the second). tutents).
Whichever glycosidation method is used in oligo- The following are examples of oligosaccharide synthe-
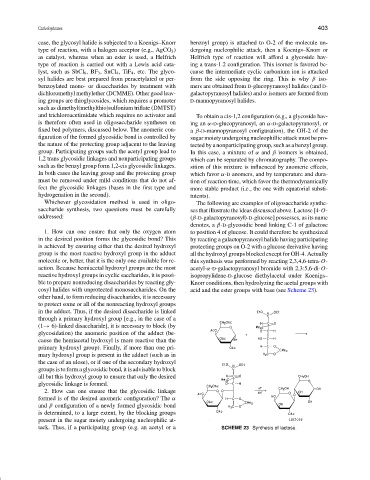
saccharide synthesis, two questions must be carefully ses that illustrate the ideas discussed above. Lactose [4-O-
addressed: (β-D-galactopyranosyl)-D-glucose] possesses, as its name
denotes, a β-D-glycosidic bond linking C-1 of galactose
1. How can one ensure that only the oxygen atom to position 4 of glucose. It could therefore be synthesized
in the desired position forms the glycosidic bond? This by reacting a galactopyranosyl halide having participating
is achieved by ensuring either that the desired hydroxyl protecting groups on O-2 with a glucose derivative having
group is the most reactive hydroxyl group in the adduct all the hydroxyl groups blocked except for OH-4. Actually
molecule or, better, that it is the only one available for re- this synthesis was performed by reacting 2,3,4,6-tetra-O-
action. Because hemiacetal hydroxyl groups are the most acetyl-α-D-galactopyranosyl bromide with 2,3:5,6-di-O-
reactive hydroxyl groups in cyclic saccharides, it is possi- isopropylidene-D-glucose diethylacetal under Koenigs–
ble to prepare nonreducing disaccharides by reacting gly- Knorr conditions, then hydrolyzing the acetal groups with
cosyl halides with unprotected monosaccharides. On the acid and the ester groups with base (see Scheme 23).
other hand, to form reducing disaccharides, it is necessary
to protect some or all of the nonreacting hydroxyl groups
in the adduct. Thus, if the desired disaccharide is linked
through a primary hydroxyl group [e.g., in the case of a
(1→ 6)-linked disaccharide], it is necessary to block (by
glycosidation) the anomeric position of the adduct (be-
cause the hemiacetal hydroxyl is more reactive than the
primary hydroxyl group). Finally, if more than one pri-
mary hydroxyl group is present in the adduct (such as in
the case of an ulose), or if one of the secondary hydroxyl
groups is to form a glycosidic bond, it is advisable to block
all but this hydroxyl group to ensure that only the desired
glycosidic linkage is formed.
2. How can one ensure that the glycosidic linkage
formed is of the desired anomeric configuration? The α
and β configuration of a newly formed glycosidic bond
is determined, to a large extent, by the blocking groups
present in the sugar moiety undergoing nucleophilic at-
tack. Thus, if a participating group (e.g. an acetyl or a SCHEME 23 Synthesis of lactose.