Page 94 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 94
P1: LLL/LLL P2: FJU Final Pages
Encyclopedia of Physical Science and Technology EN002C-80 May 25, 2001 20:18
402 Carbohydrates
B. Oligosaccharide Synthesis
Three types of oligosaccharide synthesis will be discussed
in this section; to choose between them one should con-
sider the complexity of the oligosaccharide, the length of
the proposed scheme, and the availability of its starting
materials. The synthetic methods are
1. The standard chemical method, which usually
involves a nucleophilic substitution of a leaving
group, previously introduced on a mono- or
oligosaccharide, by an appropriate saccharide adduct.
2. The same chemical reactions described in (1), but
carried on a solid support. The operation resembles
the automated synthesis of peptides and
polynucleotides carried out fixed beds of polymer or
resin. In the present case, the nonreducing end of the
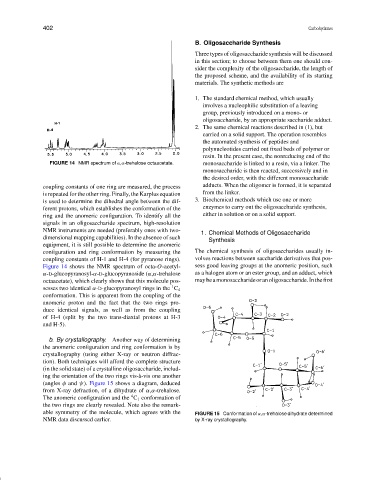
FIGURE 14 NMR spectrum of α,α-trehalose octaacetate. monosaccharide is linked to a resin, via a linker. The
monosaccharide is then reacted, successively and in
the desired order, with the different monosaccharide
coupling constants of one ring are measured, the process adducts. When the oligomer is formed, it is separated
is repeated for the other ring. Finally, the Karplus equation from the linker.
is used to determine the dihedral angle between the dif- 3. Biochemical methods which use one or more
ferent protons, which establishes the conformation of the enzymes to carry out the oligosaccharide synthesis,
ring and the anomeric configuration. To identify all the either in solution or on a solid support.
signals in an oligosaccharide spectrum, high-resolution
NMR instruments are needed (preferably ones with two-
1. Chemical Methods of Oligosaccharide
dimensional mapping capabilities). In the absence of such
Synthesis
equipment, it is still possible to determine the anomeric
configuration and ring conformation by measuring the The chemical synthesis of oligosaccharides usually in-
coupling constants of H-1 and H-4 (for pyranose rings). volves reactions between saccharide derivatives that pos-
Figure 14 shows the NMR spectrum of octa-O-acetyl- sess good leaving groups at the anomeric position, such
α-D-glucopyranosyl-α-D-glucopyranoside (α,α-trehalose as a halogen atom or an ester group, and an adduct, which
octaacetate), which clearly shows that this molecule pos- maybeamonosaccharideoranoligosaccharide.Inthefirst
1
sesses two identical α-D-glucopyranosyl rings in the C 4
conformation. This is apparent from the coupling of the
anomeric proton and the fact that the two rings pro-
duce identical signals, as well as from the coupling
of H-4 (split by the two trans-diaxial protons at H-3
and H-5).
b. By crystallography. Another way of determining
the anomeric configuration and ring conformation is by
crystallography (using either X-ray or neutron diffrac-
tion). Both techniques will afford the complete structure
(in the solid state) of a crystalline oligosaccharide, includ-
ing the orientation of the two rings vis-`a-vis one another
(angles φ and ψ). Figure 15 shows a diagram, deduced
from X-ray defraction, of a dihydrate of α,α-trehalose.
4
The anomeric configuration and the C 1 conformation of
the two rings are clearly revealed. Note also the remark-
able symmetry of the molecule, which agrees with the FIGURE 15 Conformation of α,α-trehalose dihydrate determined
NMR data discussed earlier. by X-ray crystallography.