Page 91 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 91
P1: LLL/LLL P2: FJU Final Pages
Encyclopedia of Physical Science and Technology EN002C-80 May 25, 2001 20:18
Carbohydrates 399
hydrolysate will reveal that one monomer (A in A–B–A
and B in B–A–B) occurs in double the amount of the
other; this monomer must be terminally located in the
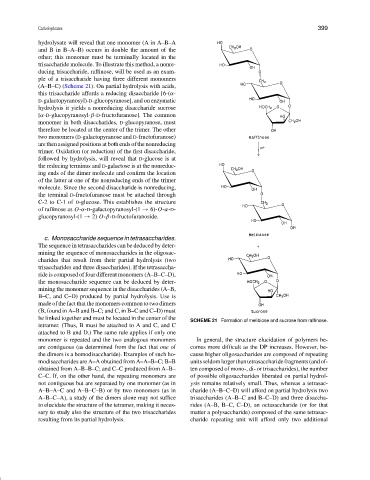
trisaccharide molecule. To illustrate this method, a nonre-
ducing trisaccharide, raffinose, will be used as an exam-
ple of a trisaccharide having three different monomers
(A–B–C) (Scheme 21). On partial hydrolysis with acids,
this trisaccharide affords a reducing disaccharide [6-(α-
D-galactopyranosyl)-D-glucopyranose], and on enzymatic
hydrolysis it yields a nonreducing disaccharide sucrose
[α-D-glucopyranosyl-β-D-fructofuranose]. The common
monomer in both disaccharides, D-glucopyranose, must
therefore be located at the center of the trimer. The other
two monomers (D-galactopyranose and D-fructofuranose)
are then assigned positions at both ends of the nonreducing
trimer. Oxidation (or reduction) of the first disaccharide,
followed by hydrolysis, will reveal that D-glucose is at
the reducing terminus and D-galactose is at the nonreduc-
ing ends of the dimer molecule and confirm the location
of the latter at one of the nonreducing ends of the trimer
molecule. Since the second disaccharide is nonreducing,
the terminal D-fructofuranose must be attached through
C-2 to C-1 of D-glucose. This establishes the structure
of raffinose as O-α-D-galactopyranosyl-(1 → 6)-O-α-D-
glucopyranosyl-(1 → 2) O-β-D-fructofuranoside.
c. Monosaccharide sequence in tetrasaccharides.
The sequence in tetrasaccharides can be deduced by deter-
mining the sequence of monosaccharides in the oligosac-
charides that result from their partial hydrolysis (two
trisaccharides and three disaccharides). If the tetrasaccha-
ride is composed of four different monomers (A–B–C–D),
the monosaccharide sequence can be deduced by deter-
mining the monomer sequence in the disaccharides (A–B,
B–C, and C–D) produced by partial hydrolysis. Use is
made of the fact that the monomers common to two dimers
(B, found in A–B and B–C; and C, in B–C and C–D) must
be linked together and must be located in the center of the
SCHEME 21 Formation of melibiose and sucrose from raffinose.
tetramer. (Thus, B must be attached to A and C, and C
attached to B and D.) The same rule applies if only one
monomer is repeated and the two analogous monomers In general, the structure elucidation of polymers be-
are contiguous (as determined from the fact that one of comes more difficult as the DP increases. However, be-
the dimers is a homodisaccharide). Examples of such ho- cause higher oligosaccharides are composed of repeating
modisaccharides are A–A obtained from A–A–B–C; B–B unitsseldomlargerthantetrasaccharidefragments(andof-
obtained from A–B–B–C; and C–C produced from A–B– ten composed of mono-, di- or trisaccharides), the number
C–C. If, on the other hand, the repeating monomers are of possible oligosaccharides liberated on partial hydrol-
not contiguous but are separated by one monomer (as in ysis remains relatively small. Thus, whereas a tetrasac-
A–B–A–C and A–B–C–B) or by two monomers (as in charide (A–B–C–D) will afford on partial hydrolysis two
A–B–C–A), a study of the dimers alone may not suffice trisaccharides (A–B–C and B–C–D) and three disaccha-
to elucidate the structure of the tetramer, making it neces- rides (A–B, B–C, C–D), an octasaccharide (or for that
sary to study also the structure of the two trisaccharides matter a polysaccharide) composed of the same tetrasac-
resulting from its partial hydrolysis. charide repeating unit will afford only two additional