Page 99 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 99
P1: LLL/LLL P2: FJU Final Pages
Encyclopedia of Physical Science and Technology EN002C-80 May 25, 2001 20:18
Carbohydrates 407
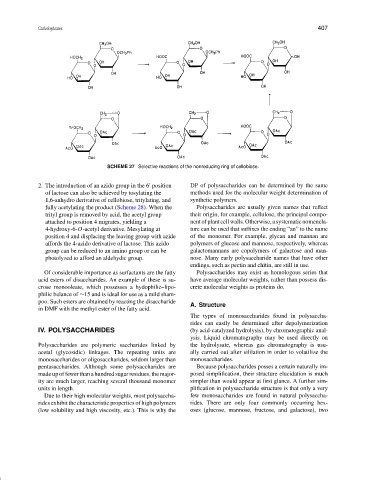
SCHEME 27 Selective reactions of the nonreducing ring of cellobiose.
2. The introduction of an azido group in the 6 position DP of polysaccharides can be determined by the same
of lactose can also be achieved by tosylating the methods used for the molecular weight determination of
1,6-anhydro derivative of cellobiose, tritylating, and synthetic polymers.
fully acetylating the product (Scheme 28). When the Polysaccharides are usually given names that reflect
trityl group is removed by acid, the acetyl group their origin, for example, cellulose, the principal compo-
attached to position 4 migrates, yielding a nent of plant cell walls. Otherwise, a systematic nomencla-
4-hydroxy-6-O-acetyl derivative. Mesylating at ture can be used that suffixes the ending “an” to the name
position 4 and displacing the leaving group with azide of the monomer. For example, glycan and mannan are
affords the 4-azido derivative of lactose. This azido polymers of glucose and mannose, respectively, whereas
group can be reduced to an amino group or can be galactomannans are copolymers of galactose and man-
photolyzed to afford an aldehydic group. nose. Many early polysaccharide names that have other
endings, such as pectin and chitin, are still in use.
Of considerable importance as surfactants are the fatty Polysaccharides may exist as homologous series that
acid esters of disaccharides. An example of these is su- have average molecular weights, rather than possess dis-
crose monooleate, which possesses a hydophilic–lipo- crete molecular weights as proteins do.
philic balance of ∼15 and is ideal for use as a mild sham-
poo. Such esters are obtained by reacting the disaccharide
A. Structure
in DMF with the methyl ester of the fatty acid.
The types of monosaccharides found in polysaccha-
rides can easily be determined after depolymerization
IV. POLYSACCHARIDES (by acid-catalyzed hydrolysis), by chromatographic anal-
ysis. Liquid chromatography may be used directly on
Polysaccharides are polymeric saccharides linked by the hydrolysate, whereas gas chromatography is usu-
acetal (glycosidic) linkages. The repeating units are ally carried out after sililation in order to volatilize the
monosaccharides or oligosaccharides, seldom larger than monosaccharides.
pentasaccharides. Although some polysaccharides are Because polysaccharides posses a certain naturally im-
made up of fewer than a hundred sugar residues, the major- posed simplification, their structure elucidation is much
ity are much larger, reaching several thousand monomer simpler than would appear at first glance. A further sim-
units in length. plification in polysaccharide structure is that only a very
Due to their high molecular weights, most polysaccha- few monosaccharides are found in natural polysaccha-
rides exhibit the characteristic properties of high polymers rides. There are only four commonly occurring hex-
(low solubility and high viscosity, etc.). This is why the oses (glucose, mannose, fructose, and galactose), two