Page 118 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 118
P1: FPP Revised Pages
Encyclopedia of Physical Science and Technology EN002C-85 May 17, 2001 20:35
466 Catalysis, Homogeneous
coordinates to a transition metal, so will trans-2-butene,
but cis-2-butene won’t. If a bare metal atom coordinates
to cis-2-butene the complex has a mirror plane, and
hence the complex is not chiral. In summary, with few
exceptions, complexation of a metal to the one face of an
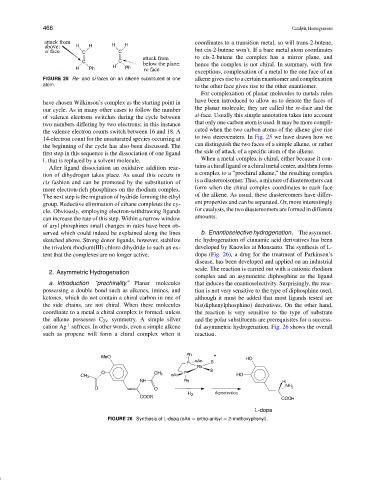
FIGURE 25 Re- and si-faces on an alkene substituted at one alkene gives rise to a certain enantiomer and complexation
atom. to the other face gives rise to the other enantiomer.
For complexation of planar molecules to metals rules
have chosen Wilkinson’s complex as the starting point in have been introduced to allow us to denote the faces of
our cycle. As in many other cases to follow the number the planar molecule; they are called the re-face and the
of valence electrons switches during the cycle between si-face. Usually this simple annotation takes into account
two numbers differing by two electrons; in this instance that only one carbon atom is used. It may be more compli-
the valence electron counts switch between 16 and 18. A cated when the two carbon atoms of the alkene give rise
14-electron count for the unsaturated species occurring at to two stereocenters. In Fig. 25 we have drawn how we
the beginning of the cycle has also been discussed. The can distinguish the two faces of a simple alkene, or rather
first step in this sequence is the dissociation of one ligand the side of attack of a specific atom of the alkene.
L that is replaced by a solvent molecule. When a metal complex is chiral, either because it con-
After ligand dissociation an oxidative addition reac- tains a chiral ligand or a chiral metal center, and then forms
tion of dihydrogen takes place. As usual this occurs in a complex to a “prochiral alkene,” the resulting complex
cis fashion and can be promoted by the substitution of is a diastereoisomer. Thus, a mixture of diastereomers can
more electron-rich phosphines on the rhodium complex. form when the chiral complex coordinates to each face
The next step is the migration of hydride forming the ethyl of the alkene. As usual, these diastereomers have differ-
ent properties and can be separated. Or, more interestingly
group. Reductive elimination of ethane completes the cy-
for catalysis, the two diastereomers are formed in different
cle. Obviously, employing electron-withdrawing ligands
amounts.
can increase the rate of this step. Within a narrow window
of aryl phosphines small changes in rates have been ob-
served which could indeed be explained along the lines b. Enantioselective hydrogenation. Theasymmet-
sketched above. Strong donor ligands, however, stabilize ric hydrogenation of cinnamic acid derivatives has been
the trivalent rhodium(III) chloro dihydride to such an ex- developed by Knowles at Monsanto. The synthesis of L-
tent that the complexes are no longer active. dopa (Fig. 26), a drug for the treatment of Parkinson’s
disease, has been developed and applied on an industrial
scale. The reaction is carried out with a cationic rhodium
2. Asymmetric Hydrogenation
complex and an asymmetric diphosphine as the ligand
a. Introduction “prochirality.” Planar molecules that induces the enantioselectivity. Surprisingly, the reac-
possessing a double bond such as alkenes, imines, and tion is not very sensitive to the type of diphosphine used,
ketones, which do not contain a chiral carbon in one of although it must be added that most ligands tested are
the side chains, are not chiral. When these molecules bis(diphenylphosphino) derivatives. On the other hand,
coordinate to a metal a chiral complex is formed, unless the reaction is very sensitive to the type of substrate
the alkene possesses C 2V symmetry. A simple silver and the polar substituents are prerequisites for a success-
+
cation Ag suffices. In other words, even a simple alkene ful asymmetric hydrogenation. Fig. 26 shows the overall
such as propene will form a chiral complex when it reaction.
FIGURE 26 Synthesis of L-dopa (oAn = ortho-anisyl = 2-methoxyphenyl).