Page 121 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 121
P1: FPP Revised Pages
Encyclopedia of Physical Science and Technology EN002C-85 May 17, 2001 20:35
Catalysis, Homogeneous 469
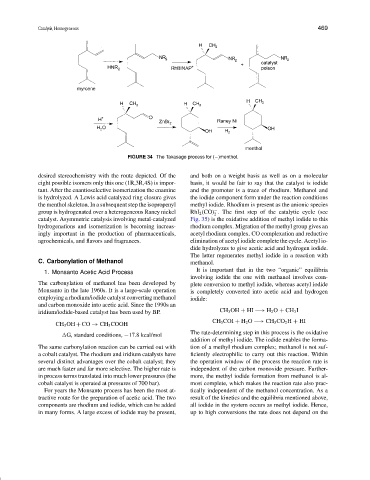
FIGURE 34 The Takasago process for (−)menthol.
desired stereochemistry with the route depicted. Of the and both on a weight basis as well as on a molecular
eight possible isomers only this one (1R,3R,4S) is impor- basis, it would be fair to say that the catalyst is iodide
tant. After the enantioselective isomerization the enamine and the promoter is a trace of rhodium. Methanol and
is hydrolyzed. A Lewis acid catalyzed ring closure gives the iodide component form under the reaction conditions
the menthol skeleton. In a subsequent step the isopropenyl methyl iodide. Rhodium is present as the anionic species
−
group is hydrogenated over a heterogeneous Raney nickel RhI 2 (CO) . The first step of the catalytic cycle (see
2
catalyst. Asymmetric catalysis involving metal-catalyzed Fig. 35) is the oxidative addition of methyl iodide to this
hydrogenations and isomerization is becoming increas- rhodium complex. Migration of the methyl group gives an
ingly important in the production of pharmaceuticals, acetyl rhodium complex. CO complexation and reductive
agrochemicals, and flavors and fragrances. elimination of acetyl iodide complete the cycle. Acetyl io-
dide hydrolyzes to give acetic acid and hydrogen iodide.
The latter regenerates methyl iodide in a reaction with
C. Carbonylation of Methanol methanol.
It is important that in the two “organic” equilibria
1. Monsanto Acetic Acid Process
involving iodide the one with methanol involves com-
The carbonylation of methanol has been developed by plete conversion to methyl iodide, whereas acetyl iodide
Monsanto in the late 1960s. It is a large-scale operation is completely converted into acetic acid and hydrogen
employing a rhodium/iodide catalyst converting methanol iodide:
and carbon monoxide into acetic acid. Since the 1990s an
iridium/iodide-based catalyst has been used by BP. CH 3 OH + HI −→ H 2 O + CH 3 I
CH 3 COI + H 2 O −→ CH 3 CO 2 H + HI
CH 3 OH + CO → CH 3 COOH
The rate-determining step in this process is the oxidative
G, standard conditions, −17.8 kcal/mol
addition of methyl iodide. The iodide enables the forma-
The same carbonylation reaction can be carried out with tion of a methyl rhodium complex; methanol is not suf-
a cobalt catalyst. The rhodium and iridium catalysts have ficiently electrophilic to carry out this reaction. Within
several distinct advantages over the cobalt catalyst; they the operation window of the process the reaction rate is
are much faster and far more selective. The higher rate is independent of the carbon monoxide pressure. Further-
in process terms translated into much lower pressures (the more, the methyl iodide formation from methanol is al-
cobalt catalyst is operated at pressures of 700 bar). most complete, which makes the reaction rate also prac-
For years the Monsanto process has been the most at- tically independent of the methanol concentration. As a
tractive route for the preparation of acetic acid. The two result of the kinetics and the equilibria mentioned above,
components are rhodium and iodide, which can be added all iodide in the system occurs as methyl iodide. Hence,
in many forms. A large excess of iodide may be present, up to high conversions the rate does not depend on the