Page 119 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 119
P1: FPP Revised Pages
Encyclopedia of Physical Science and Technology EN002C-85 May 17, 2001 20:35
Catalysis, Homogeneous 467
FIGURE 27 Diastereomeric alkene complexes.
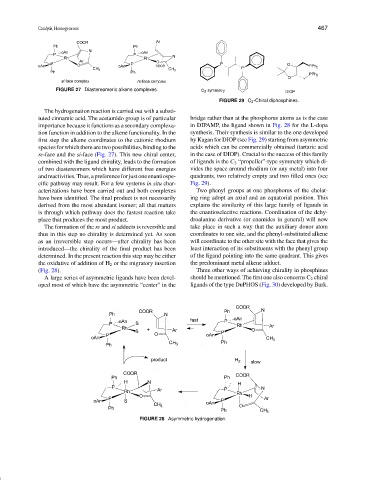
FIGURE 29 C 2 -Chiral diphosphines.
The hydrogenation reaction is carried out with a substi-
tuted cinnamic acid. The acetamido group is of particular bridge rather than at the phosphorus atoms as is the case
importance because it functions as a secondary complexa- in DIPAMP, the ligand shown in Fig. 28 for the L-dopa
tion function in addition to the alkene functionality. In the synthesis. Their synthesis is similar to the one developed
first step the alkene coordinates to the cationic rhodium by Kagan for DIOP (see Fig. 29) starting from asymmetric
species for which there are two possibilities, binding to the acids which can be commercially obtained (tartaric acid
re-face and the si-face (Fig. 27). This new chiral center, in the case of DIOP). Crucial to the success of this family
combined with the ligand chirality, leads to the formation of ligands is the C 2 “propeller”-type symmetry which di-
of two diastereomers which have different free energies vides the space around rhodium (or any metal) into four
andreactivities.Thus,apreferenceforjustoneenantiospe- quadrants, two relatively empty and two filled ones (see
cific pathway may result. For a few systems in situ char- Fig. 29).
acterizations have been carried out and both complexes Two phenyl groups at one phosphorus of the chelat-
have been identified. The final product is not necessarily ing ring adopt an axial and an equatorial position. This
derived from the most abundant isomer; all that matters explains the similarity of this large family of ligands in
is through which pathway does the fastest reaction take the enantioselective reactions. Coordination of the dehy-
place that produces the most product. droalanine derivative (or enamides in general) will now
The formation of the re and si adducts is reversible and take place in such a way that the auxiliary donor atom
thus in this step no chirality is determined yet. As soon coordinates to one site, and the phenyl-substituted alkene
as an irreversible step occurs—after chirality has been will coordinate to the other site with the face that gives the
introduced—the chirality of the final product has been least interaction of its substituents with the phenyl group
determined. In the present reaction this step may be either of the ligand pointing into the same quadrant. This gives
the oxidative of addition of H 2 or the migratory insertion the predominant metal alkene adduct.
(Fig. 28). Three other ways of achieving chirality in phosphines
A large series of asymmetric ligands have been devel- should be mentioned. The first one also concerns C 2 chiral
oped most of which have the asymmetric “center” in the ligands of the type DuPHOS (Fig. 30) developed by Burk.
FIGURE 28 Asymmetric hydrogenation.