Page 124 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 124
P1: FPP Revised Pages
Encyclopedia of Physical Science and Technology EN002C-85 May 17, 2001 20:35
472 Catalysis, Homogeneous
will not be hydroformylated. The 2-alkene concentration position for the remaining four ligands, carbon monox-
will increase and the 1-alkene concentration will decrease; ide, phosphorus ligands, and the alkene substrate. As
this will slow down the rate of the hydroformylation re- usual, the ligand replacement equilibria have been sim-
action. This makes the rhodium catalyst less suited for plified. After an insertion step a 16-electron species is
the conversion of alkenes other than propene for which formed which restores its 18-electron count with a new
isomerization is irrelevant. To date, hydroformylation of CO molecule. There is consensus in the literature that the
higher alkenes is industrially still carried out with cobalt catalytic species with two phosphine ligands plays a ma-
catalysts. jor role, certainly when L = PPh 3 . The high preference for
Propene hydroformylation can be done yielding a lin- linear aldehyde formation is ascribed to steric factors—
earity ranging from 60 to 95% dependent on the phosphine congestion at the rhodium center favors the formation of
concentration. At very high phosphine concentration the linear alkyl and acyl species. Especially when two phos-
rate is low, but the linearity achieves its maximum value. phine ligands coordinate bis-equatorially, there is a strong
The commercial process operates presumably around 30 preference for the formation of n-alkyl groups. Later we
◦
bar of syn-gas, at 120 C, at high phosphine concentra- will see how one can make use of this concept to obtain
tions, and linearities around 92%. The estimated turnover highly selective catalysts.
frequency of moles of product per mole of rhodium com- For rhodium-phosphine catalysts, the kinetics shows
plex per hour is in the order of 300. Low ligand concentra- that one of the first steps, complexation of alkene or migra-
tions, with concomitant low linearities, will give turnover tory insertion of the alkene, is the rate-determining step. A
frequencies in the order of 10,000 at 10 bar and 90 C. first order in alkene is observed, while there is a negative
◦
In the presence of carbon monoxide this rhodium cata- dependence in the CO and/or phosphine concentration,
lyst has no activity for hydrogenation and the selectivity which also stems from the replacement reaction. The rate
based on starting material is virtually 100%. The n-butanal of reaction is independent of the hydrogen concentration.
produced contains no alcohol and can be converted both Another limiting case is the hydroformylation us-
to butanol, to 2-ethyl-hexanol-1, and to other products as ing electron-poor rhodium catalysts (e.g., HRh(CO) 4 or
desired. HRh(CO) 3 L, L being an electron-poor phosphite). In this
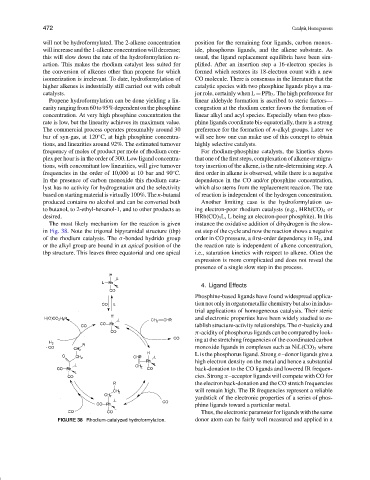
The most likely mechanism for the reaction is given instance the oxidative addition of dihydrogen is the slow-
in Fig. 38. Note the trigonal bipyramidal structure (tbp) est step of the cycle and now the reaction shows a negative
of the rhodium catalysts. The σ-bonded hydrido group order in CO pressure, a first-order dependency in H 2 , and
or the alkyl group are bound in an apical position of the the reaction rate is independent of alkene concentration,
tbp structure. This leaves three equatorial and one apical i.e., saturation kinetics with respect to alkene. Often the
expression is more complicated and does not reveal the
presence of a single slow step in the process.
4. Ligand Effects
Phosphine-based ligands have found widespread applica-
tionnotonlyinorganometallicchemistrybutalsoinindus-
trial applications of homogeneous catalysis. Their steric
and electronic properties have been widely studied to es-
tablish structure-activity relationships. The σ-basicity and
π-acidity of phosphorus ligands can be compared by look-
ing at the stretching frequencies of the coordinated carbon
monoxide ligands in complexes such as NiL(CO) 3 where
L is the phosphorus ligand. Strong σ–donor ligands give a
high electron density on the metal and hence a substantial
back-donation to the CO ligands and lowered IR frequen-
cies. Strong π–acceptor ligands will compete with CO for
the electron back-donation and the CO stretch frequencies
will remain high. The IR frequencies represent a reliable
yardstick of the electronic properties of a series of phos-
phine ligands toward a particular metal.
Thus, the electronic parameter for ligands with the same
FIGURE 38 Rhodium-catalyzed hydroformylation. donor atom can be fairly well measured and applied in a