Page 147 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 147
P1: GTY/GWT P2: GLM Final
Encyclopedia of Physical Science and Technology EN006K-933 June 29, 2001 12:14
Fuel Chemistry 257
and therefore the time required for heterogeneous com-
bustion of solid residue (char) is shorter. The drying time
of a small particle is the time required to heat the particle
to the boiling point of the water and evaporate the wa-
ter. This depends on the amount of water in the particle,
particle size, and the heating rate of the particle. The rate
of heat transfer to the particle depends on the tempera-
ture difference between the boiler furnace and the particle
temperature.
Devolatilization of a fuel particle involves thermal de-
composition of the organic structure, thereby releasing the
resulting fragments and products. The rate of devolatiliza-
tion depends on the rate of heat transfer to the particle to
break weak bonds resulting in the primary decomposition
of products. The primary products of decomposition travel
from the interior of the particle to the surface through the
pores of the solid. While doing so, the primary products,
depending on their nature, could react with each other or
with the char surface and result in secondary products or
deposits on the walls of the pores. The devolatilization
◦
process begins at about 350 C and is a strong function
of temperature. The products consist of water, hydrogen-
rich gases and vapors (hydrocarbons), carbon oxides, tars,
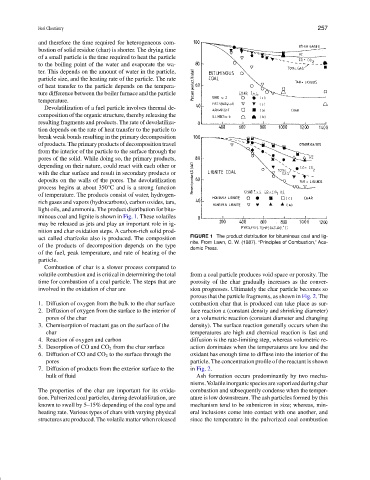
light oils, and ammonia. The product distribution for bitu-
minous coal and lignite is shown in Fig. 1. These volatiles
may be released as jets and play an important role in ig-
nition and char oxidation steps. A carbon-rich solid prod-
uct called char/coke also is produced. The composition FIGURE 1 The product distribution for bituminous coal and lig-
nite. From Lawn, C. W. (1987). “Principles of Combustion,” Aca-
of the products of decomposition depends on the type
demic Press.
of the fuel, peak temperature, and rate of heating of the
particle.
Combustion of char is a slower process compared to
volatile combustion and is critical in determining the total from a coal particle produces void space or porosity. The
time for combustion of a coal particle. The steps that are porosity of the char gradually increases as the conver-
involved in the oxidation of char are sion progresses. Ultimately the char particle becomes so
porous that the particle fragments, as shown in Fig. 2. The
1. Diffusion of oxygen from the bulk to the char surface combustion char that is produced can take place as sur-
2. Diffusion of oxygen from the surface to the interior of face reaction a (constant density and shrinking diameter)
pores of the char or a volumetric reaction (constant diameter and changing
3. Chemisorption of reactant gas on the surface of the density). The surface reaction generally occurs when the
char temperatures are high and chemical reaction is fast and
4. Reaction of oxygen and carbon diffusion is the rate-limiting step, whereas volumetric re-
5. Desorption of CO and CO 2 from the char surface action dominates when the temperatures are low and the
6. Diffusion of CO and CO 2 to the surface through the oxidant has enough time to diffuse into the interior of the
pores particle. The concentration profile of the reactant is shown
7. Diffusion of products from the exterior surface to the in Fig. 2.
bulk of fluid Ash formation occurs predominantly by two mecha-
nisms.Volatileinorganicspeciesarevaporizedduringchar
The properties of the char are important for its oxida- combustion and subsequently condense when the temper-
tion. Pulverized coal particles, during devolatilization, are ature is low downstream. The ash particles formed by this
known to swell by 5–15% depending of the coal type and mechanism tend to be submicron in size; whereas, min-
heating rate. Various types of chars with varying physical eral inclusions come into contact with one another, and
structures are produced. The volatile matter when released since the temperature in the pulverized coal combustion