Page 198 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 198
P1: LLL/GVX P2: GQT Final Pages
Encyclopedia of Physical Science and Technology EN011G-539 July 14, 2001 21:48
Organic Chemical Systems, Theory 445
If the original orbitals φ 1 and φ 2 are degenerate,
ε = 0,
they will enter the new orbitals ψ 1 and ψ 2 with equal
weights. If the initial energies are unequal, the more stable
initial orbital φ 1 will enter with a numerically larger coef-
ficient into the more stable resulting orbital ψ 1 and with a
smaller coefficient into the less stable resulting orbital ψ 2 .
The opposite will be true for the less stable initial orbital
φ 2 . This result is quite logical: In the more stable of the
two orbitals, the electrons spend more time on the more
electronegative atom. 2
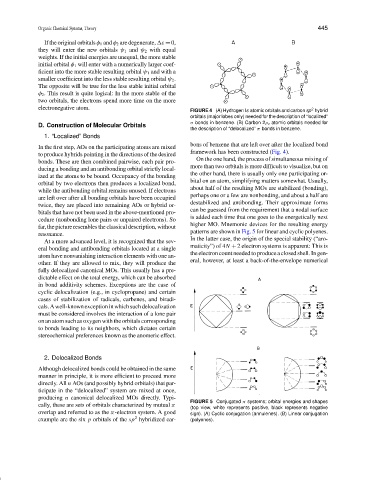
FIGURE 4 (A) Hydrogen Is atomic orbitals and carbon sp hybrid
orbitals (major lobes only) needed for the description of “localized”
σ bonds in benzene. (B) Carbon 2ρ z atomic orbitals needed for
D. Construction of Molecular Orbitals
the description of “delocalized” π bonds in benzene.
1. “Localized” Bonds
bons of benzene that are left over after the localized bond
In the first step, AOs on the participating atoms are mixed
framework has been constructed (Fig. 4).
to produce hybrids pointing in the directions of the desired
On the one hand, the process of simultaneous mixing of
bonds. These are then combined pairwise, each pair pro-
more than two orbitals is more difficult to visualize, but on
ducing a bonding and an antibonding orbital strictly local-
the other hand, there is usually only one participating or-
ized at the atoms to be bound. Occupancy of the bonding
bital on an atom, simplifying matters somewhat. Usually,
orbital by two electrons then produces a localized bond,
about half of the resulting MOs are stabilized (bonding),
while the antibonding orbital remains unused. If electrons
perhaps one or a few are nonbonding, and about a half are
are left over after all bonding orbitals have been occupied
twice, they are placed into remaining AOs or hybrid or- destabilized and antibonding. Their approximate forms
can be guessed from the requirement that a nodal surface
bitals that have not been used in the above-mentioned pro-
is added each time that one goes to the energetically next
cedure (nonbonding lone pairs or unpaired electrons). So
higher MO. Mnemonic devices for the resulting energy
far, the picture resembles the classical description, without
patterns are shown in Fig. 5 for linear and cyclic polyenes.
resonance.
In the latter case, the origin of the special stability (“aro-
At a more advanced level, it is recognized that the sev-
maticity”)of4N + 2 electron systems is apparent: This is
eral bonding and antibonding orbitals located at a single
the electron count needed to produce a closed shell. In gen-
atom have nonvanishing interaction elements with one an-
eral, however, at least a back-of-the-envelope numerical
other. If they are allowed to mix, they will produce the
fully delocalized canonical MOs. This usually has a pre-
dictable effect on the total energy, which can be absorbed
in bond additivity schemes. Exceptions are the case of
cyclic delocalization (e.g., in cyclopropane) and certain
cases of stabilization of radicals, carbenes, and biradi-
cals.Awell-knownexceptioninwhichsuchdelocalization
must be considered involves the interaction of a lone pair
on an atom such as oxygen with the orbitals corresponding
to bonds leading to its neighbors, which dictates certain
stereochemical preferences known as the anomeric effect.
2. Delocalized Bonds
Although delocalized bonds could be obtained in the same
manner in principle, it is more efficient to proceed more
directly. All n AOs (and possibly hybrid orbitals) that par-
ticipate in the “delocalized” system are mixed at once,
producing n canonical delocalized MOs directly. Typi-
FIGURE 5 Conjugated π systems; orbital energies and shapes
cally, these are sets of orbitals characterized by mutual π
(top view, white represents positive, black represents negative
overlap and referred to as the π-electron system. A good sign). (A) Cyclic conjugation (annulenes). (B) Linear conjugation
2
example are the six p orbitals of the sp hybridized car- (polyenes).