Page 40 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 40
P1: LDK Revised Pages
Encyclopedia of Physical Science and Technology EN001F-21 May 7, 2001 13:44
Alkaloids 479
TABLE I Historical Data on Isolation, Structure Elucidation, and Synthesis of Some Well-Known Al-
kaloids
Isolation of the Elucidation of the Determination of the
Alkaloid Source pure alkaloid correct structure absolute configuration Synthesis
Morphine Opium 1805 1925 1955 1952
Emetine Psychotria ipecacuanha 1817 1948 1959 1950
Strychnine Strychnos nux-vomica 1818 1946 1956 1954
Atropine Atropa belladonna 1819 1901 1933 1903
Quinine Cinchona bark(s) 1820 1907 1950 1944
Coniine Conium maculatum 1827 1881 1932 1886
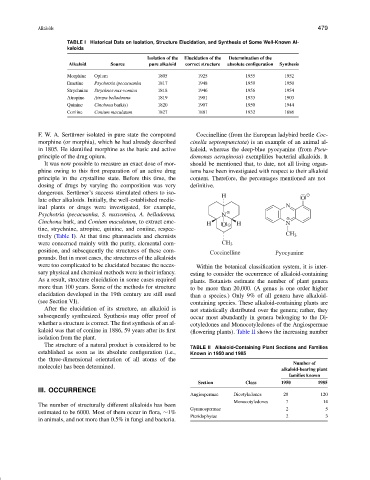
F. W. A. Sert¨urner isolated in pure state the compound Coccinelline (from the European ladybird beetle Coc-
morphine (or morphia), which he had already described cinella septempunctata) is an example of an animal al-
in 1805. He identified morphine as the basic and active kaloid, whereas the deep-blue pyocyanine (from Pseu-
principle of the drug opium. domonas aeruginosa) exemplifies bacterial alkaloids. It
It was now possible to measure an exact dose of mor- should be mentioned that, to date, not all living organ-
phine owing to this first preparation of an active drug isms have been investigated with respect to their alkaloid
principle in the crystalline state. Before this time, the content. Therefore, the percentages mentioned are not
dosing of drugs by varying the composition was very definitive.
dangerous. Sert¨urner’s success stimulated others to iso-
H
late other alkaloids. Initially, the well-established medic- O
inal plants or drugs were investigated, for example, N
Psychotria ipecacuanha, S. nuxvomica, A. belladonna, N
Cinchona bark, and Conium maculatum, to extract eme- H O H N
tine, strychnine, atropine, quinine, and coniine, respec-
tively (Table I). At that time pharmacists and chemists CH 3
were concerned mainly with the purity, elemental com- CH 3
position, and subsequently the structures of these com- Coccinelline Pyocyanine
pounds. But in most cases, the structures of the alkaloids
were too complicated to be elucidated because the neces- Within the botanical classification system, it is inter-
sary physical and chemical methods were in their infancy. esting to consider the occurrence of alkaloid-containing
As a result, structure elucidation in some cases required plants. Botanists estimate the number of plant genera
more than 100 years. Some of the methods for structure to be more than 20,000. (A genus is one order higher
elucidation developed in the 19th century are still used than a species.) Only 9% of all genera have alkaloid-
(see Section VI). containing species. These alkaloid-containing plants are
After the elucidation of its structure, an alkaloid is not statistically distributed over the genera; rather, they
subsequently synthesized. Synthesis may offer proof of occur most abundantly in genera belonging to the Di-
whether a structure is correct. The first synthesis of an al- cotyledones and Monocotyledones of the Angiospermae
kaloid was that of coniine in 1886, 59 years after its first (flowering plants). Table II shows the increasing number
isolation from the plant.
The structure of a natural product is considered to be
TABLE II Alkaloid-Containing Plant Sections and Families
established as soon as its absolute configuration (i.e., Known in 1950 and 1985
the three-dimensional orientation of all atoms of the
Number of
molecule) has been determined.
alkaloid-bearing plant
families known
Section Class 1950 1985
III. OCCURRENCE
Angiospermae Dicotyledones 28 120
Monocotyledones 7 14
The number of structurally different alkaloids has been
Gymnospermae 2 5
estimated to be 6000. Most of them occur in flora, ∼1%
Pteridophytae 2 3
in animals, and not more than 0.5% in fungi and bacteria.