Page 41 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 41
P1: LDK Revised Pages
Encyclopedia of Physical Science and Technology EN001F-21 May 7, 2001 13:44
480 Alkaloids
of plant families known to contain alkaloids and the dom- V. EXTRACTION, ISOLATION,
inance of the flowering plants. Alkaloids have rarely been PURIFICATION, AND ANALYSIS
found in the Gymnospermae and Pteridophytae.
It has been suggested that in 40% of all plant families, A. Physical and Chemical Features
at least one alkaloid-containing species is known. On the of Alkaloids
other hand, there are plant families (e.g., Papaveraceae)
As with other organic molecules, the physical properties
in which all species contain alkaloids. Consequently, the
of alkaloids strongly depend on their molecular structures.
distribution of the alkaloids in flora is very dissimilar.
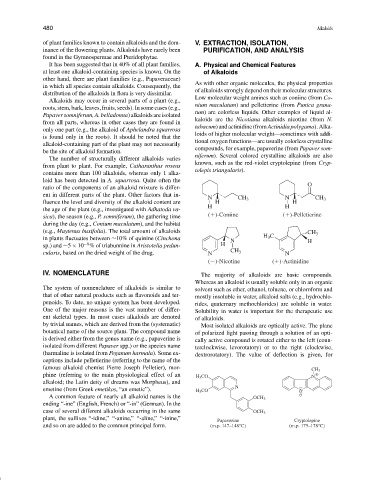
Low molecular weight amines such as coniine (from Co-
Alkaloids may occur in several parts of a plant (e.g.,
nium maculatum) and pelletierine (from Punica grana-
roots, stem, bark, leaves, fruits, seeds). In some cases (e.g.,
tum) are colorless liquids. Other examples of liquid al-
Papaversomniferum,A.belladonna)alkaloidsareisolated
kaloids are the Nicotiana alkaloids nicotine (from N.
from all parts, whereas in other cases they are found in
tabacum)andactinidine(fromActinidiapolygama).Alka-
only one part (e.g., the alkaloid of Aphelandra squarrosa
loids of higher molecular weight—sometimes with addi-
is found only in the roots). It should be noted that the
tional oxygen functions—are usually colorless crystalline
alkaloid-containing part of the plant may not necessarily
compounds, for example, papaverine (from Papaver som-
be the site of alkaloid formation.
niferum). Several colored crystalline alkaloids are also
The number of structurally different alkaloids varies
known, such as the red-violet cryptolepine (from Cryp-
from plant to plant. For example, Catharanthus roseus
tolepis triangularis).
contains more than 100 alkaloids, whereas only 1 alka-
loid has been detected in A. squarrosa. Quite often the
O
ratio of the components of an alkaloid mixture is differ-
ent in different parts of the plant. Other factors that in-
N CH 3 N CH 3
fluence the level and diversity of the alkaloid content are H H
H H
the age of the plant (e.g., investigated with Adhatoda va-
sica), the season (e.g., P. somniferum), the gathering time ( )-Coniine ( )-Pelletierine
during the day (e.g., Conium maculatum), and the habitat
(e.g., Maytenus buxifolia). The total amount of alkaloids CH 3
H 3 C
in plants fluctuates between ∼10% of quinine (Cinchona N H
−6
sp.) and ∼5 × 10 % of triabunnine in Aristotelia pedun- H
cularis, based on the dried weight of the drug. N CH 3 N
( )-Nicotine ( )-Actinidine
IV. NOMENCLATURE The majority of alkaloids are basic compounds.
Whereas an alkaloid is usually soluble only in an organic
The system of nomenclature of alkaloids is similar to solvent such as ether, ethanol, toluene, or chloroform and
that of other natural products such as flavonoids and ter- mostly insoluble in water, alkaloid salts (e.g., hydrochlo-
penoids. To date, no unique system has been developed. rides, quaternary methochlorides) are soluble in water.
One of the major reasons is the vast number of differ- Solubility in water is important for the therapeutic use
ent skeletal types. In most cases alkaloids are denoted of alkaloids.
by trivial names, which are derived from the (systematic) Most isolated alkaloids are optically active. The plane
botanical name of the source plant. The compound name of polarized light passing through a solution of an opti-
is derived either from the genus name (e.g., papaverine is cally active compound is rotated either to the left (coun-
isolated from different Papaver spp.) or the species name terclockwise, levorotatory) or to the right (clockwise,
(harmaline is isolated from Peganum harmala). Some ex- dextrorotatory). The value of deflection is given, for
ceptions include pelletierine (referring to the name of the
famous alkaloid chemist Pierre Joseph Pelletier), mor-
CH 3
phine (referring to the main physiological effect of an H 3 CO N
alkaloid; the Latin deity of dreams was Morpheus), and
emetine (from Greek emetikos, “an emetic”). H 3 CO N N
A common feature of nearly all alkaloid names is the OCH 3
ending “-ine” (English, French) or “-in” (German). In the
case of several different alkaloids occurring in the same OCH 3
plant, the suffixes “-idine,”“-anine,”“-aline,”“-inine,”
Papaverine Cryptolepine
and so on are added to the common principal form. (m.p. 147–148°C) (m.p. 175–178°C)