Page 44 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 44
P1: LDK Revised Pages
Encyclopedia of Physical Science and Technology EN001F-21 May 7, 2001 13:44
Alkaloids 483
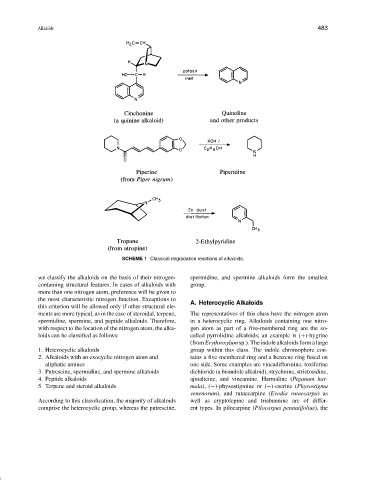
SCHEME 1 Classical degradation reactions of alkaloids.
we classify the alkaloids on the basis of their nitrogen- spermidine, and spermine alkaloids form the smallest
containing structural features. In cases of alkaloids with group.
more than one nitrogen atom, preference will be given to
the most characteristic nitrogen function. Exceptions to
A. Heterocyclic Alkaloids
this criterion will be allowed only if other structural ele-
ments are more typical, as in the case of steroidal, terpene, The representatives of this class have the nitrogen atom
spermidine, spermine, and peptide alkaloids. Therefore, in a heterocyclic ring. Alkaloids containing one nitro-
with respect to the location of the nitrogen atom, the alka- gen atom as part of a five-membered ring are the so-
loids can be classified as follows: called pyrrolidine alkaloids; an example is (+)-hygrine
(from Erythroxylum sp.). The indole alkaloids form a large
1. Heterocyclic alkaloids group within this class. The indole chromophore con-
2. Alkaloids with an exocyclic nitrogen atom and tains a five-membered ring and a benzene ring fused on
aliphatic amines one side. Some examples are vincadifformine, toxiferine
3. Putrescine, spermidine, and spermine alkaloids dichloride (a bisindole alkaloid), strychnine, strictosidine,
4. Peptide alkaloids ajmalicine, and vincamine. Harmaline (Peganum har-
5. Terpene and steroid alkaloids mala), (−)-physostigmine or (−)-eserine (Physostigma
venenosum), and rutaecarpine (Evodia rutaecarpa)as
According to this classification, the majority of alkaloids well as cryptolepine and triabunnine are of differ-
comprise the heterocyclic group, whereas the putrescine, ent types. In pilocarpine (Pilocarpus pennatifolius), the