Page 48 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 48
P1: LDK Revised Pages
Encyclopedia of Physical Science and Technology EN001F-21 May 7, 2001 13:44
Alkaloids 487
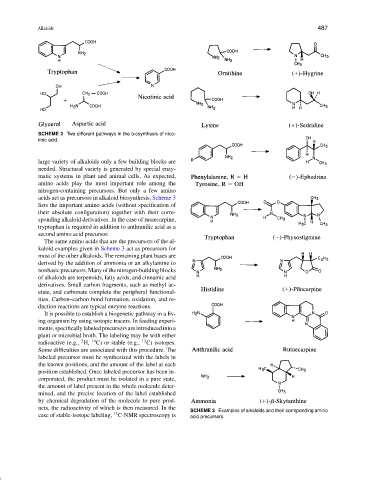
SCHEME 2 Two different pathways in the biosynthesis of nico-
tinic acid.
large variety of alkaloids only a few building blocks are
needed. Structural variety is generated by special enzy-
matic systems in plant and animal cells. As expected,
amino acids play the most important role among the
nitrogen-containing precursors. But only a few amino
acids act as precursors in alkaloid biosynthesis. Scheme 3
lists the important amino acids (without specification of
their absolute configuration) together with their corre-
sponding alkaloid derivatives. In the case of rutaecarpine,
tryptophan is required in addition to anthranilic acid as a
second amino acid precursor.
The same amino acids that are the precursors of the al-
kaloid examples given in Scheme 3 act as precursors for
most of the other alkaloids. The remaining plant bases are
derived by the addition of ammonia or an alkylamine to
nonbasicprecursors.Manyofthenitrogen-buildingblocks
of alkaloids are terpenoids, fatty acids, and cinnamic acid
derivatives. Small carbon fragments, such as methyl ac-
etate, and carbonate complete the peripheral functional-
ities. Carbon–carbon bond formation, oxidation, and re-
duction reactions are typical enzyme reactions.
It is possible to establish a biogenetic pathway in a liv-
ing organism by using isotopic tracers. In feeding experi-
ments, specifically labeled precursors are introduced into a
plant or microbial broth. The labeling may be with either
3
radioactive (e.g., H, 14 C) or stable (e.g., 13 C) isotopes.
Some difficulties are associated with this procedure. The
labeled precursor must be synthesized with the labels in
the known positions, and the amount of the label at each
position established. Once labeled precursor has been in-
corporated, the product must be isolated in a pure state,
the amount of label present in the whole molecule deter-
mined, and the precise location of the label established
by chemical degradation of the molecule to pure prod-
ucts, the radioactivity of which is then measured. In the SCHEME 3 Examples of alkaloids and their corrsponding amino
13
case of stable-isotope labeling, C-NMR spectroscopy is acid precursors.