Page 50 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 50
P1: LDK Revised Pages
Encyclopedia of Physical Science and Technology EN001F-21 May 7, 2001 13:44
Alkaloids 489
(mandrake). Therefore, a classification of plant species atoms (e.g., —COOCH 3 → —H) and/or opening or form-
based on the structures of their alkaloids and other chem- ing of chemical bonds. In Scheme 6 examples of struc-
ical ingredients has been attempted. Such a system of tural changes of strictosidine leading to the indole alka-
classification of plant species by chemical compounds is loids ajmalicine, strychnine, and vincamine are given. The
known as plant chemosystematics or chemotaxonomy. structural changes occur only in the secologanin part of
To investigate a group of alkaloids in the chemosystem- strictosidine: in all cases the tryptamine part remains un-
atic sense it is necessary to have a large body of statistical rearranged. Therefore, to emphasize the changes in the
material available; otherwise, the results will be of uncer- secologanin part, the carbon skeleton of the latter is pre-
tain value. sented in heavy lines in the scheme. These changes can
The distribution of indole alkaloids in plant species has occur by rotations around or rearrangements of various
been quite well investigated. The number of structurally carbon–carbon bonds. Changes of oxygen functionality
known alkaloids is ∼1200. They have been isolated from and double bonds and so on are not considered. The car-
morethan400differentplantspecies.Somealkaloidswere bon skeleton of ajmalicine can be derived from that of
isolated from only one species; others were from many strictosidine by rotation of one carbon–carbon bond. Two
different species. A total of 1200 indole alkaloids have additional rotations are necessary to explain the forma-
been isolated nearly 3500 times. These data underline the tion of the carbon skeleton of strychnine. In addition, the
importance of this class of alkaloids for chemosystematic COOCH 3 group should be eliminated and two additional
studies. To establish a botanical classification system on carbon atoms (as an active acetate unit) have to be intro-
the structural basis of the indole alkaloids plants contain, duced into the molecule to reach strychnine. To explain
it is important to investigate their biogenetic relationships. the formation of the carbon skeleton of vincamine from
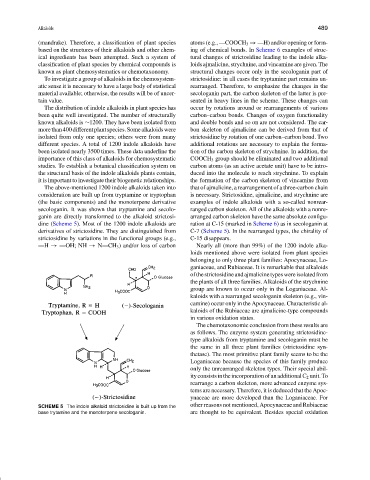
The above-mentioned 1200 indole alkaloids taken into that of ajmalicine, a rearrangement of a three-carbon chain
consideration are built up from tryptamine or tryptophan is necessary. Strictosidine, ajmalicine, and strychnine are
(the basic components) and the monoterpene derivative examples of indole alkaloids with a so-called nonrear-
secologanin. It was shown that tryptamine and secolo- ranged carbon skeleton. All of the alkaloids with a nonre-
ganin are directly transformed to the alkaloid strictosi- arranged carbon skeleton have the same absolute configu-
dine (Scheme 5). Most of the 1200 indole alkaloids are ration at C-15 (marked in Scheme 6) as in secologanin at
derivatives of strictosidine. They are distinguished from C-7 (Scheme 5). In the rearranged types, the chirality of
strictosidine by variations in the functional groups (e.g., C-15 disappears.
—H → —OH; NH → N—CH 3 ) and/or loss of carbon Nearly all (more than 99%) of the 1200 indole alka-
loids mentioned above were isolated from plant species
belonging to only three plant families: Apocynaceae, Lo-
ganiaceae, and Rubiaceae. It is remarkable that alkaloids
ofthestrictosidineandajmalicinetypeswereisolatedfrom
the plants of all three families. Alkaloids of the strychnine
group are known to occur only in the Loganiaceae. Al-
kaloids with a rearranged secologanin skeleton (e.g., vin-
camine) occur only in the Apocynaceae. Characteristic al-
kaloids of the Rubiaceae are ajmalicine-type compounds
in various oxidation states.
The chemotaxonomic conclusion from these results are
as follows. The enzyme system generating strictosidine-
type alkaloids from tryptamine and secologanin must be
the same in all three plant families (strictosidine syn-
thetase). The most primitive plant family seems to be the
Loganiaceae because the species of this family produce
only the unrearranged skeleton types. Their special abil-
ity consists in the incorporation of an additional C 2 unit. To
rearrange a carbon skeleton, more advanced enzyme sys-
tems are necessary. Therefore, it is deduced that the Apoc-
ynaceae are more developed than the Loganiaceae. For
SCHEME 5 The indole alkaloid strictosidine is built up from the other reasons not mentioned, Apocynaceae and Rubiaceae
base trytamine and the monoterpene secologanin. are thought to be equivalent. Besides special oxidation