Page 47 - Academic Press Encyclopedia of Physical Science and Technology 3rd Organic Chemistry
P. 47
P1: LDK Revised Pages
Encyclopedia of Physical Science and Technology EN001F-21 May 7, 2001 13:44
486 Alkaloids
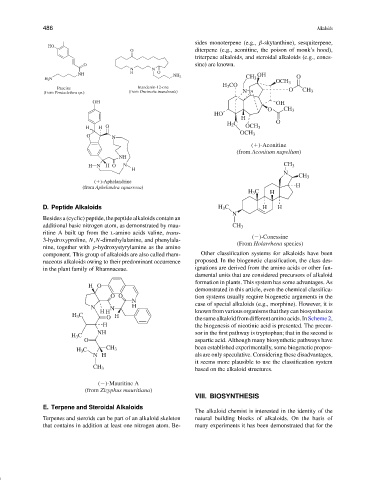
sides monoterpene (e.g., β-skytanthine), sesquiterpene,
HO
O diterpene (e.g., aconitine, the poison of monk’s hood),
triterpene alkaloids, and steroidal alkaloids (e.g., cones-
O sine) are known.
N N
NH H O
NH 2 OH
H 2 N CH 3 O
OCH 3
H 3 CO
Paucine Inandenin-12-one O
(from Pentaclethra sp.) (from Oncinotis inandensis) N CH 3
OH OH
O CH 3
HO
H
H 2 C O
H H O OCH 3
OCH 3
O N
( )-Aconitine
(from Aconitum napellum)
NH
H N H O N CH 3
H
N
CH 3
( )-Aphelandrine
(from Aphelandra squarrosa) H
H 3 C H
D. Peptide Alkaloids H 3 C H H
N
Besides a (cyclic) peptide, the peptide alkaloids contain an
additional basic nitrogen atom, as demonstrated by mau- CH 3
ritine A built up from the L-amino acids valine, trans-
( )-Conessine
3-hydroxyproline, N,N-dimethylalanine, and phenylala-
(From Holarrhena species)
nine, together with p-hydroxystyrylamine as the amino
component. This group of alkaloids are also called rham- Other classification systems for alkaloids have been
naceous alkaloids owing to their predominant occurrence proposed. In the biogenetic classification, the class des-
in the plant family of Rhamnaceae. ignations are derived from the amino acids or other fun-
damental units that are considered precursors of alkaloid
formation in plants. This system has some advantages. As
H O
demonstrated in this article, even the chemical classifica-
O O tion systems usually require biogenetic arguments in the
N
N N H case of special alkaloids (e.g., morphine). However, it is
H H known from various organisms that they can biosynthesize
H 3 C H
O the same alkaloid from different amino acids. In Scheme 2,
H the biogenesis of nicotinic acid is presented. The precur-
NH sor in the first pathway is tryptophan; that in the second is
H 3 C
O aspartic acid. Although many biosynthetic pathways have
been established experimentally, some biogenetic propos-
H 3 C CH 3
N H als are only speculative. Considering these disadvantages,
it seems more plausible to use the classification system
CH 3
based on the alkaloid structures.
( )-Mauritine A
(from Zizyphus mauritiana)
VIII. BIOSYNTHESIS
E. Terpene and Steroidal Alkaloids
The alkaloid chemist is interested in the identity of the
Terpenes and steroids can be part of an alkaloid skeleton natural building blocks of alkaloids. On the basis of
that contains in addition at least one nitrogen atom. Be- many experiments it has been demonstrated that for the