Page 106 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 106
P1: GRB Final Pages
Encyclopedia of Physical Science and Technology EN005M-206 June 15, 2001 20:25
184 Electrochemistry
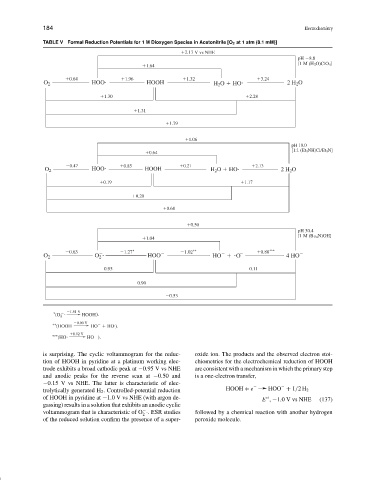
TABLE V Formal Reduction Potentials for 1 M Dioxygen Species in Acetonitrile [O 2 at 1 atm (8.1 mM)]
2.17 V vs NHE
pH 8.8
[1 M (H 3 O)ClO 4 ]
1.64
0.64 1.96 1.32 3.24
O 2 HOO HOOH H 2 O HO 2 H 2 O
1.30 2.28
1.31
1.79
1.06
pH 10.0
[1:1 (Et 3 NH)Cl/Et 3 N]
0.64
0.47 0.85 0.21 2.13
O 2 HOO HOOH H 2 O HO 2 H 2 O
0.19 1.17
0.20
0.68
0.50
pH 30.4
[1 M (Bu 4 N)OH]
1.04
∗ ∗∗ ∗∗∗
0.63 1.27 1.02 0.80
O 2 O 2 HOO HO O 4 HO
0.95 0.11
0.90
0.53
∗ 1.51 V ).
(O 2 HOOH
∗∗ (HOOH 0.90 V HO HO ).
∗∗∗ (HO 0.92 V HO ).
is surprising. The cyclic voltammogram for the reduc- oxide ion. The products and the observed electron stoi-
tion of HOOH in pyridine at a platinum working elec- chiometries for the electrochemical reduction of HOOH
trode exhibits a broad cathodic peak at −0.95VvsNHE are consistent with a mechanism in which the primary step
and anodic peaks for the reverse scan at −0.50 and is a one-electron transfer,
−0.15 V vs NHE. The latter is characteristic of elec-
−
trolytically generated H 2 . Controlled-potential reduction HOOH + e − HOO + 1/2H 2
of HOOH in pyridine at −1.0 V vs NHE (with argon de- E , −1.0 V vs NHE (137)
◦
gassing) results in a solution that exhibits an anodic cyclic
−
voltammogram that is characteristic of O ·. ESR studies followed by a chemical reaction with another hydrogen
2
of the reduced solution confirm the presence of a super- peroxide molecule.