Page 110 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 110
P1: GRB Final Pages
Encyclopedia of Physical Science and Technology EN005M-206 June 15, 2001 20:25
188 Electrochemistry
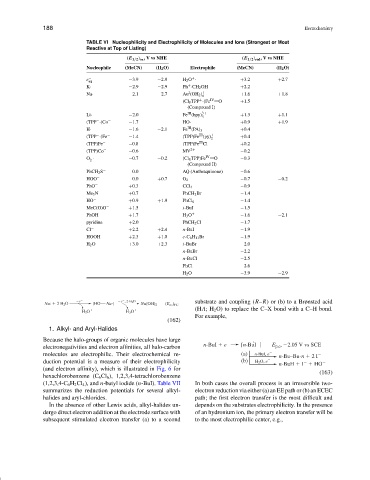
TABLE VI Nucleophilicity and Electrophilicity of Molecules and Ions (Strongest or Most
Reactive at Top of Listing)
(E 1/2 ) ox ,VvsNHE (E 1/2 ) red ,VvsNHE
Nucleophile (MeCN) (H 2 O) Electrophile (MeCN) (H 2 O)
+
e − −3.9 −2.9 H 2 O · +3.2 +2.7
aq
+
K· −2.9 −2.9 Ph ·CH 2 OH +2.2
I
Na· −2.1 −2.7 Au (OH 2 ) + +1.6 +1.8
6
+
(Cl 8 TPP ·)Fe IV O +1.5
(Compound I)
III
Li· −2.0 Fe (bpy) 3+ +1.3 +1.1
3
(TPP ·)Co − −1.7 HO· +0.9 +1.9
−
III
H· −1.6 −2.1 Fe (PA) 3 +0.4
III
−
(TPP ·)Fe − −1.4 (TPP)Fe (py) + 2 +0.4
III
(TPP)Fe − −0.8 (TPP)Fe Cl +0.2
(TPP)Co − −0.6 MV 2+ −0.2
− IV
O · −0.7 −0.2 (Cl 8 TPP)Fe O −0.3
2
(Compound II)
PhCH 2 S − 0.0 AQ (Anthraquinone) −0.6
HOO − 0.0 +0.7 O 2 −0.7 −0.2
PhO − +0.3 CCl 4 −0.9
Me 3 N +0.7 PhCH 2 Br −1.4
HO − +0.9 +1.9 PhCl 6 −1.4
MeC(O)O − +1.5 t-BuI −1.5
PhOH +1.7 H 3 O + −1.6 −2.1
pyridine +2.0 PhCH 2 Cl −1.7
Cl − +2.2 +2.4 n-BuI −1.9
HOOH +2.3 +1.0 c-C 6 H 11 Br −1.9
H 2 O +3.0 +2.3 t-BuBr −2.0
n-BuBr −2.2
n-BuCl −2.5
PhCl −2.6
H 2 O −3.9 −2.9
e e , 2 H 2 O substrate and coupling (R–R) or (b) to a Brønsted acid
Nu: 2 H 2 O [HO Nu ] Nu(OH) 2 (E ox ) EC
H 3 O H 3 O (HA;H 2 O) to replace the C–X bond with a C–H bond.
For example,
(162)
1. Alkyl- and Aryl-Halides
Because the halo-groups of organic molecules have large
n-BuI e [n-BuI ] E p,c , 2.05 V vs SCE
electronegativities and electron affinities, all halo-carbon
molecules are electrophilic. Their electrochemical re- (a) n-BuI, e n-Bu–Bu-n 2 I
duction potential is a measure of their electrophilicity (b) H 2 O, e n-BuH I HO
(and electron affinity), which is illustrated in Fig. 6 for
(163)
hexachlorobenzene (C 6 Cl 6 ), 1,2,3,4-tetrachlorobenzene
(1,2,3,4-C 6 H 2 Cl 4 ), and n-butyl iodide (n-BuI). Table VII In both cases the overall process is an irreversible two-
summarizes the reduction potentials for several alkyl- electron reduction via either (a) an EE path or (b) an ECEC
halides and aryl-chlorides. path; the first electron transfer is the most difficult and
In the absence of other Lewis acids, alkyl-halides un- depends on the substrates electrophilicity. In the presence
dergo direct electron addition at the electrode surface with of an hydronium ion, the primary electron transfer will be
subsequent stimulated electron transfer (a) to a second to the most electrophilic center, e.g.,