Page 154 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 154
P1: LLL/LPB P2: FJU Final Pages
Encyclopedia of Physical Science and Technology EN005F220 June 15, 2001 20:44
396 Elemental Analysis, Organic Compounds
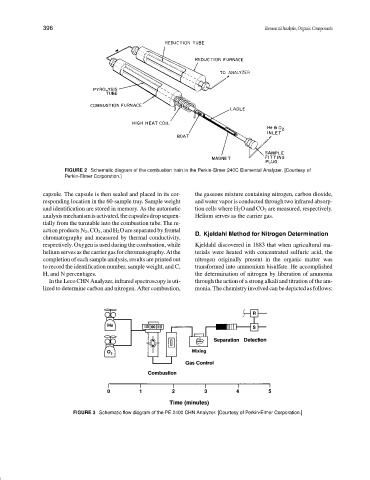
FIGURE 2 Schematic diagram of the combustion train in the Perkin-Elmer 240C Elemental Analyzer. [Courtesy of
Perkin-Elmer Corporation.]
capsule. The capsule is then sealed and placed in its cor- the gaseous mixture containing nitrogen, carbon dioxide,
responding location in the 60-sample tray. Sample weight and water vapor is conducted through two infrared absorp-
and identification are stored in memory. As the automatic tion cells where H 2 O and CO 2 are measured, respectively.
analysismechanismisactivated,thecapsulesdropsequen- Helium serves as the carrier gas.
tially from the turntable into the combustion tube. The re-
action products N 2 ,CO 2 , and H 2 O are separated by frontal
D. Kjeldahl Method for Nitrogen Determination
chromatography and measured by thermal conductivity,
respectively. Oxygen is used during the combustion, while Kjeldahl discovered in 1883 that when agricultural ma-
helium serves as the carrier gas for chromatography. At the terials were heated with concentrated sulfuric acid, the
completion of each sample analysis, results are printed out nitrogen originally present in the organic matter was
to record the identification number, sample weight, and C, transformed into ammonium bisulfate. He accomplished
H, and N percentages. the determination of nitrogen by liberation of ammonia
In the Leco CHN Analyzer, infrared spectroscopy is uti- through the action of a strong alkali and titration of the am-
lized to determine carbon and nitrogen. After combustion, monia. The chemistry involved can be depicted as follows:
FIGURE 3 Schematic flow diagram of the PE 2400 CHN Analyzer. [Courtesy of Perkin-Elmer Corporation.]