Page 215 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 215
P1: ZCK/MBQ P2: GQT Final Pages
Encyclopedia of Physical Science and Technology EN008B-382 June 30, 2001 18:58
682 Liquid Chromatography
reliability but also the lower cost. The most common com-
mercial LC/MS instrument is based on the electrospray
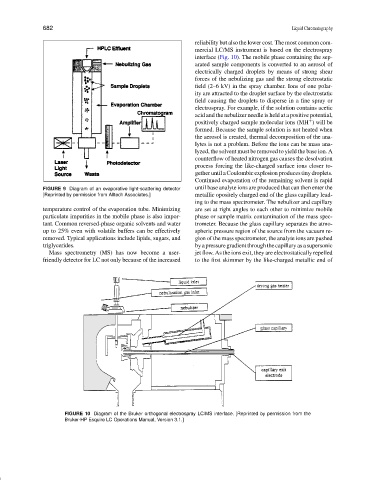
interface (Fig. 10). The mobile phase containing the sep-
arated sample components is converted to an aerosol of
electrically charged droplets by means of strong shear
forces of the nebulizing gas and the strong electrostatic
field (2–6 kV) in the spray chamber. Ions of one polar-
ity are attracted to the droplet surface by the electrostatic
field causing the droplets to disperse in a fine spray or
electrospray. For example, if the solution contains acetic
acid and the nebulizer needle is held at a positive potential,
positively charged sample molecular ions (MH ) will be
+
formed. Because the sample solution is not heated when
the aerosol is created, thermal decomposition of the ana-
lytes is not a problem. Before the ions can be mass ana-
lyzed, the solvent must be removed to yield the base ion. A
counterflow of heated nitrogen gas causes the desolvation
process forcing the like-charged surface ions closer to-
gether until a Coulombic explosion produces tiny droplets.
Continued evaporation of the remaining solvent is rapid
FIGURE 9 Diagram of an evaporative light-scattering detector until base analyte ions are produced that can then enter the
[Reprinted by permission from Alltech Associates.] metallic opositely charged end of the glass capillary lead-
ing to the mass spectrometer. The nebulizer and capillary
temperature control of the evaporation tube. Minimizing are set at right angles to each other to minimize mobile
particulate impurities in the mobile phase is also impor- phase or sample matrix contamination of the mass spec-
tant. Common reversed-phase organic solvents and water trometer. Because the glass capillary separates the atmo-
up to 25% even with volatile buffers can be effectively spheric pressure region of the source from the vacuum re-
removed. Typical applications include lipids, sugars, and gion of the mass spectrometer, the analyte ions are pushed
triglycerides. by a pressure gradient through the capillary as a supersonic
Mass spectrometry (MS) has now become a user- jet flow. As the ions exit, they are electrostatically repelled
friendly detector for LC not only because of the increased to the first skimmer by the like-charged metallic end of
FIGURE 10 Diagram of the Bruker orthogonal electrospray LC/MS interface. [Reprinted by permission from the
Bruker-HP Esquire LC Operations Manual, Version 3.1.]