Page 219 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 219
P1: ZCK/MBQ P2: GQT Final Pages
Encyclopedia of Physical Science and Technology EN008B-382 June 30, 2001 18:58
686 Liquid Chromatography
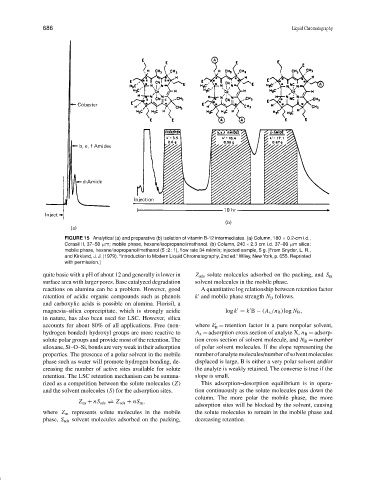
FIGURE 15 Analytical (a) and preparative (b) isolation of vitamin B-12 intermediates. (a) Column, 180 × 0.2-cm i.d.,
Corasil II, 37–50 µm; mobile phase, hexane/isopropanol/methanol. (b) Column, 240 × 2.3 cm i.d. 37–80 µm silica:
mobile phase, hexane/isopropanol/methanol (5 : 2 : 1), flow rate 34 ml/min; injected sample, 5 g. [From Snyder, L. R.,
and Kirkland, J. J. (1979). “Introduction to Modern Liquid Chromatography, 2nd ed.” Wiley, New York, p. 655. Reprinted
with permission.]
quite basic with a pH of about 12 and generally is lower in Z ads solute molecules adsorbed on the packing, and S m
surface area with larger pores. Base catalyzed degradation solvent molecules in the mobile phase.
reactions on alumina can be a problem. However, good A quantitative log relationship between retention factor
retention of acidic organic compounds such as phenols k and mobile phase strength N B follows.
and carboxylic acids is possible on alumina. Florisil, a
magnesia–silica coprecipitate, which is strongly acidic log k = k B − (A x /n B ) log N B ,
in nature, has also been used for LSC. However, silica
accounts for about 80% of all applications. Free (non- where k = retention factor in a pure nonpolar solvent,
B
hydrogen bonded) hydroxyl groups are more reactive to A x = adsorption cross section of analyte X, n B = adsorp-
solute polar groups and provide most of the retention. The tion cross section of solvent molecule, and N B = number
siloxane, Si–O–Si, bonds are very weak in their adsorption of polar solvent molecules. If the slope representing the
properties. The presence of a polar solvent in the mobile numberofanalytemolecules/numberofsolventmolecules
phase such as water will promote hydrogen bonding, de- displaced is large, B is either a very polar solvent and/or
creasing the number of active sites available for solute the analyte is weakly retained. The converse is true if the
retention. The LSC retention mechanism can be summa- slope is small.
rized as a competition between the solute molecules (Z) This adsorption–desorption equilibrium is in opera-
and the solvent molecules (S) for the adsorption sites. tion continuously as the solute molecules pass down the
column. The more polar the mobile phase, the more
Z m + nS ads
Z ads + nS m ,
adsorption sites will be blocked by the solvent, causing
where Z m represents solute molecules in the mobile the solute molecules to remain in the mobile phase and
phase, S ads solvent molecules adsorbed on the packing, decreasing retention.