Page 222 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 222
P1: ZCK/MBQ P2: GQT Final Pages
Encyclopedia of Physical Science and Technology EN008B-382 June 30, 2001 18:58
Liquid Chromatography 689
carried out in the presence of water with the usual resultant
formation of a polymeric layer:
O O R
H 2 O
Si OH R 2 SiCl 2 Si (Si O) n H
O O R
These reactions are often difficult to control because both
cross-linking and linear polymerization are possible. The
polymer layer may be too thick to permit good chromato-
graphic mass transfer or too thin to give adequate sample
capacity. In addition, residual silanols will be formed if
not all the Si Cl groups react; an end capping reaction
with TMCS is recommended.
Alternatively, bifunctional chlorosilanes with an
ether bridging group or simply sterically protected
monochlorosilanes such as chlorodiisopropyloctyl silane
have both provided protection of the siloxane bond be-
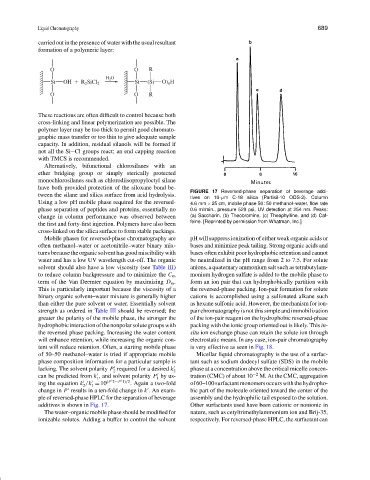
FIGURE 17 Reversed-phase separation of beverage addi-
tween the silane and silica surface from acid hydrolysis.
tives on 10-µm C-18 silica (Partisil-10 ODS-2). Column
Using a low pH mobile phase required for the reversed- 4.6 mm × 25 cm, mobile phase 50 : 50 methanol–water, flow rate
phase separation of peptides and proteins, essentially no 0.6 ml/min., pressure 529 psi, UV detection at 254 nm. Peaks:
change in column performance was observed between (a) Saccharin, (b) Theobromine, (c) Theophylline, and (d) Caf-
the first and forty-first injection. Polymers have also been feine. [Reprinted by permission from Whatman, Inc.]
cross-linked on the silica surface to form stable packings.
Mobile phases for reversed-phase chromatography are pH will suppress ionization of either weak organic acids or
often methanol–water or acetonitrile–water binary mix- bases and minimize peak tailing. Strong organic acids and
tures because the organic solvent has good miscibility with bases often exhibit poor hydrophobic retention and cannot
water and has a low UV wavelength cut-off. The organic be neutralized in the pH range from 2 to 7.5. For solute
solvent should also have a low viscosity (see Table III) anions, a quaternary ammonium salt such as tetrabutylam-
monium hydrogen sulfate is added to the mobile phase to
to reduce column backpressure and to minimize the C m
term of the Van Deemter equation by maximizing D m . form an ion pair that can hydrophobically partition with
This is particularly important because the viscosity of a the reversed-phase packing. Ion-pair formation for solute
binary organic solvent–water mixture is generally higher cations is accomplished using a sulfonated alkane such
than either the pure solvent or water. Essentially solvent as hexane sulfonic acid. However, the mechanism for ion-
strength as ordered in Table III should be reversed; the pair chromatography is not this simple and immobilization
greater the polarity of the mobile phase, the stronger the of the ion-pair reagent on the hydrophobic reversed-phase
hydrophobicinteractionofthenonpolarsolutegroupswith packing with the ionic group oriented out is likely. This in-
the reversed phase packing. Increasing the water content situ ion exchange phase can retain the solute ion through
will enhance retention, while increasing the organic con- electrostatic means. In any case, ion-pair chromatography
tent will reduce retention. Often, a starting mobile phase is very effective as seen in Fig. 18.
of 50–50 methanol–water is tried if appropriate mobile Micellar liquid chromatography is the use of a surfac-
phase composition information for a particular sample is tant such as sodium dodecyl sulfate (SDS) in the mobile
lacking. The solvent polarity P required for a desired k
phase at a concentration above the critical micelle concen-
2 2
can be predicted from k , and solvent polarity P by us- tration (CMC) of about 10 −2 M. At the CMC, aggregation
1 1
ing the equation k /k = 10 (P 2−P 1)/2 . Again a two-fold of60–100surfactantmonomersoccurswiththehydropho-
2 1
change in P results in a ten-fold change in k . An exam- bic part of the molecule oriented toward the center of the
ple of reversed-phase HPLC for the separation of beverage assembly and the hydrophilic tail exposed to the solution.
additives is shown in Fig. 17. Other surfactants used have been cationic or nonionic in
The water–organic mobile phase should be modified for nature, such as cetyltrimethylammonium ion and Brij-35,
ionizable solutes. Adding a buffer to control the solvent respectively. For reversed-phase HPLC, the surfactant can