Page 226 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 226
P1: ZCK/MBQ P2: GQT Final Pages
Encyclopedia of Physical Science and Technology EN008B-382 June 30, 2001 18:58
Liquid Chromatography 693
In past years, the direct detection of inorganic anions,
cations, and small aliphatic organic acids and bases af-
ter column separation has been difficult. Development
of ion chromatography in the mid 1970s solved this
problem. Now two approaches, both using low-capacity
ion-exchange columns and a conductivity detector, are
commercially available. First developed by Small, the
dual-column ion chromatography system traps the ions of
the mobile phase by connecting a high-capacity suppres-
sor column downstream from the analytical ion-exchange
column. For example, using a sodium hydroxide mobile
phase, the separated anions elute into a protonated cation
suppressor column. There the mobile phase is neutralized
− + +
to water as shown in the equation Res–SO H + Na +
3
−
+
−
OH → Res–SO Na + H 2 O, and the separated an-
3
ions are changed to the corresponding acids, Res–
−
−
−
+
+
+
SO H + M + A → Res–SO M + H + A . Sensi-
−
+
3 3
tive conductivity detection of the separated ions is now
possible at the sub-ppm level. The analogous system for
cation analysis, in which HCl is the eluent and the suppres-
sor column is an anion exchanger in the hydroxide form, is
equally effective. Hollow fibers, and more recently mem-
branes, have been used in place of the suppressor column.
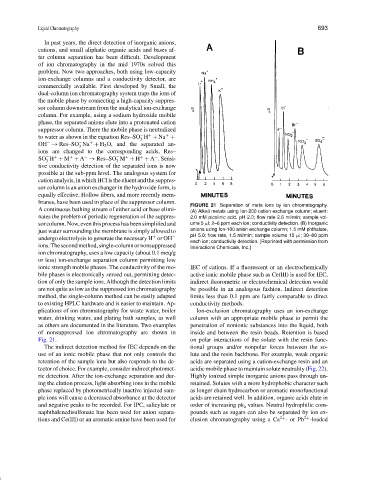
FIGURE 21 Separation of meta ions by ion chromatography.
A continuous bathing stream of either acid or base elimi- (A) Alkali metals using Ion-200 cation exchange column; eluent:
nates the problem of periodic regeneration of the suppres- 2.0 mM picolinic acid, pH 2.0; flow rate 2.6 ml/min; sample vol-
sorcolumn.Now,eventhisprocesshasbeensimplifiedand ume 5 µl; 2–6 ppm each ion; conductivity detection. (B) Inorganic
just water surrounding the membrane is simply allowed to anions using Ion-100 anion exchange column; 1.5 mM phthalate,
pH 5.0; flow rate, 1.5 ml/min; sample volume 10 µl; 30–80 ppm
undergo electrolysis to generate the necessary H or OH −
+
each ion; conductivity detection. [Reprinted with permission from
ions.Thesecondmethod,single-columnornonsuppressed Interactions Chemicals, Inc.]
ion chromatography, uses a low capacity (about 0.1 meq/g
or less) ion-exchange separation column permitting low
ionic strength mobile phases. The conductivity of the mo- IEC of cations. If a fluorescent or an electrochemically
bile phases is electronically zeroed out, permitting detec- active ionic mobile phase such as Ce(III) is used for IEC,
tion of only the sample ions. Although the detection limits indirect fluorometric or electrochemical detection would
are not quite as low as the suppressed ion chromatography be possible in an analogous fashion. Indirect detection
method, the single-column method can be easily adapted limits less than 0.1 ppm are fairly comparable to direct
to existing HPLC hardware and is easier to maintain. Ap- conductivity methods.
plications of ion chromatography for waste water, boiler Ion-exclusion chromatography uses an ion-exchange
water, drinking water, and plating bath samples, as well column with an appropriate mobile phase to permit the
as others are documented in the literature. Two examples penetration of nonionic substances into the liquid, both
of nonsuppressed ion chromatography are shown in inside and between the resin beads. Retention is based
Fig. 21. on polar interactions of the solute with the resin func-
The indirect detection method for IEC depends on the tional groups and/or nonpolar forces between the so-
use of an ionic mobile phase that not only controls the lute and the resin backbone. For example, weak organic
retention of the sample ions but also responds to the de- acids are separated using a cation-exchange resin and an
tector of choice. For example, consider indirect photomet- acidic mobile phase to maintain solute neutrality (Fig. 22).
ric detection. After the ion-exchange separation and dur- Highly ionized simple inorganic anions pass through un-
ing the elution process, light-absorbing ions in the mobile retained. Solutes with a more hydrophobic character such
phase replaced by photometrically inactive injected sam- as longer chain hydrocarbon or aromatic monofunctional
ple ions will cause a decreased absorbance at the detector acids are retained well. In addition, organic acids elute in
and negative peaks to be recorded. For IPC, salicylate or order of increasing pk a values. Neutral hydrophilic com-
naphthalenedisulfonate has been used for anion separa- pounds such as sugars can also be separated by ion ex-
2+
2+
tions and Ce(III) or an aromatic amine have been used for clusion chromatography using a Ca -orPb -loaded