Page 224 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 224
P1: ZCK/MBQ P2: GQT Final Pages
Encyclopedia of Physical Science and Technology EN008B-382 June 30, 2001 18:58
Liquid Chromatography 691
is in the field of chiral separations. Two approaches can
be employed to separate enantiomers. One method is to
derivatize the enantiomers with an optically pure chi-
ral reagent, forming two chiral centers in the products.
These diasteromers have different physical properties and
can be separated by conventional normal-phase HPLC.
The derivatizing reagent should have bulky groups at-
tached directly to the chiral center and generate deriva-
tives with the two chiral centers close to each other to
provide a more facile resolution of the diasteromers. For
example, the reagent a-methyl-p-nitro-benzylamine will
permit the resolution of racemic carboxylic acids, while
a-naphthylethylisocyanate can modify racemic alcohols
before separation.
The second approach is to use either a chiral mobile or
stationary phaseto directlydistinguish theoptical isomers.
The use of a chiral mobile phase is based on the premise FIGURE 19 Interaction between chiral stationary phase and
that the sample compounds will form strong associations amide derivative of (R)-ibuprofen. [From Braithwaite, A., and
with the chiral reagent. Based on ligand exchange chro- Smith, F. J. (1996). “Chromatographic Methods, 5th Ed.” Chap-
man & Hall, London.]
matography, D- and L-amino acids could be separated us-
ing an optically active copper (II) proline complex in the
mobile phase. If a L-proline ligand is used, the L-amino presence of an aromatic group as part of the solute struc-
acid elutes after the D-enantiomer and vice versa using the ture to ensure inclusion complexation with the glycosidic
D-proline ligand. Ion-pair formation using an optically ac- oxygens is important. The other cyclodextrins shown in
tive base such as quinine has permitted the separation of Fig. 20 either smaller or larger in size can also provide
acid enantiomers. In this case, the formation of an opti- steric chiral recognition but are not as commonly used as
cally active dynamic ion exchange resin may also assist the β form. Proteins such as bovine serum albumin (BSA)
in the separation. when bonded to silica have also been shown to provide
For chiral recognition, three simultaneous interactions, chiral recognition of low molecular compounds, such as
one of which is stereochemically based such as hydro- aromatic amino acids, coumarins and benzoin derivatives.
gen bonding, dipole–dipole, and/or dipole-induced dipole
of the stationary phase with the analytes, should occur. D. Ion-Exchange LC
The preparation and characterization of chiral stationary
phases for the separation of enantiomers by HPLC has Ion-exchange chromatography is still considered the dom-
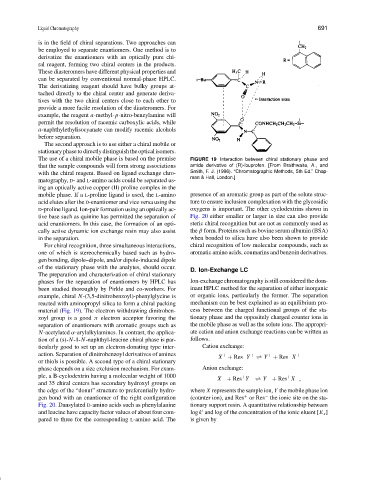
been studied thoroughly by Pirkle and co-workers. For inant HPLC method for the separation of either inorganic
example, chiral N-(3,5-dinitrobenzoyl)-phenylglycine is or organic ions, particularly the former. The separation
reacted with aminopropyl silica to form a chiral packing mechanism can be best explained as an equilibrium pro-
material (Fig. 19). The electron withdrawing dinitroben- cess between the charged functional groups of the sta-
zoyl group is a good π electron acceptor favoring the tionary phase and the oppositely charged counter ions in
separation of enantiomers with aromatic groups such as the mobile phase as well as the solute ions. The appropri-
N-acetylated α-arylalkylamines. In contrast, the applica- ate cation and anion exchange reactions can be written as
tion of a (s)-N-1-N-naphthyl-leucine chiral phase is par- follows.
ticularly good to set up an electron-donating type inter- Cation exchange:
action. Separation of dinitrobenzoyl derivatives of amines
−
X + Res Y + Y + Res X +
+
−
+
or thiols is possible. A second type of a chiral stationary
phase depends on a size exclusion mechanism. For exam- Anion exchange:
ple, a B-cyclodextrin having a molecular weight of 1000
−
−
+
−
+
X + Res Y − Y + Res X ,
and 35 chiral centers has secondary hydroxyl groups on
the edge of the “donut” structure to preferentially hydro- where X represents the sample ion, Y the mobile phase ion
gen bond with an enantiomer of the right configuration (counter ion), and Res or Res the ionic site on the sta-
−
+
Fig. 20. Dansylated D-amino acids such as phenylalanine tionary support resin. A quantitative relationship between
and leucine have capacity factor values of about four com- log k and log of the concentration of the ionic eluent [E x ]
pared to three for the corresponding L-amino acid. The is given by