Page 230 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 230
P1: ZCK/MBQ P2: GQT Final Pages
Encyclopedia of Physical Science and Technology EN008B-382 June 30, 2001 18:58
Liquid Chromatography 697
such as DRYLAB are commercially available. An ex- matic compounds in a few minutes using a short polybu-
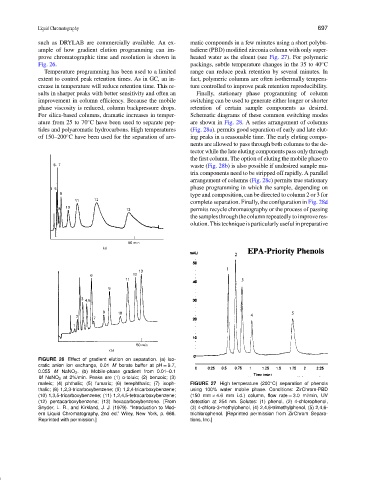
ample of how gradient elution programming can im- tadiene (PBD) modified zirconia column with only super-
prove chromatographic time and resolution is shown in heated water as the eluent (see Fig. 27). For polymeric
◦
Fig. 26. packings, subtle temperature changes in the 35 to 40 C
Temperature programming has been used to a limited range can reduce peak retention by several minutes. In
extent to control peak retention times. As in GC, an in- fact, polymeric columns are often isothermally tempera-
crease in temperature will reduce retention time. This re- ture controlled to improve peak retention reproducibility.
sults in sharper peaks with better sensitivity and often an Finally, stationary phase programming of column
improvement in column efficiency. Because the mobile switching can be used to generate either longer or shorter
phase viscosity is reduced, column backpressure drops. retention of certain sample components as desired.
For silica-based columns, dramatic increases in temper- Schematic diagrams of these common switching modes
◦
ature from 25 to 70 C have been used to separate pep- are shown in Fig. 28. A series arrangement of columns
tides and polyaromatic hydrocarbons. High temperatures (Fig. 28a). permits good separation of early and late elut-
◦
of 150–200 C have been used for the separation of aro- ing peaks in a reasonable time. The early eluting compo-
nents are allowed to pass through both columns to the de-
tector while the late eluting components pass only through
the first column. The option of eluting the mobile phase to
waste (Fig. 28b) is also possible if undesired sample ma-
trix components need to be stripped off rapidly. A parallel
arrangement of columns (Fig. 28c) permits true stationary
phase programming in which the sample, depending on
type and composition, can be directed to column 2 or 3 for
complete separation. Finally, the configuration in Fig. 28d
permits recycle chromatography or the process of passing
the samples through the column repeatedly to improve res-
olution. This technique is particularly useful in preparative
FIGURE 26 Effect of gradient elution on separation. (a) iso-
cratic anion ion exchange, 0.01 M borate buffer at pH = 9.7,
0.055 M NaNO 3 . (b) Mobile-phase gradient from 0.01–0.1
M NaNO 3 at 2%/min. Peaks are (1) o-toluic; (2) benzoic; (3)
maleic; (4) phthalic; (5) fumaric; (6) terephthalic; (7) isoph- FIGURE 27 High temperature (200 C) separation of phenols
◦
thalic; (8) 1,2,3-tricarboxybenzene; (9) 1,2,4-tricarboxybenzene; using 100% water mobile phase. Conditions: ZirChrom-PBD
(10) 1,3,5-tricarboxybenzene; (11) 1,2,4,5-tetracarboxybenzene; (150 mm × 4.6 mm i.d.) column, flow rate = 3.0 ml/min, UV
(12) pentacarboxybenzene; (13) hexacarboxybenzene. [From detection at 254 nm. Solutes: (1) phenol, (2) 4-chlorophenol,
Snyder, L. R., and Kirkland, J. J. (1979). “Introduction to Mod- (3) 4-chloro-3-methylphenol, (4) 2,4,6-trimethylphenol, (5) 2,4,6-
ern Liquid Chromatography, 2nd ed.” Wiley, New York, p. 666. trichlorophenol. [Reprinted permission from ZirChrom Separa-
Reprinted with permission.] tions, Inc.]