Page 218 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 218
P1: ZCK/MBQ P2: GQT Final Pages
Encyclopedia of Physical Science and Technology EN008B-382 June 30, 2001 18:58
Liquid Chromatography 685
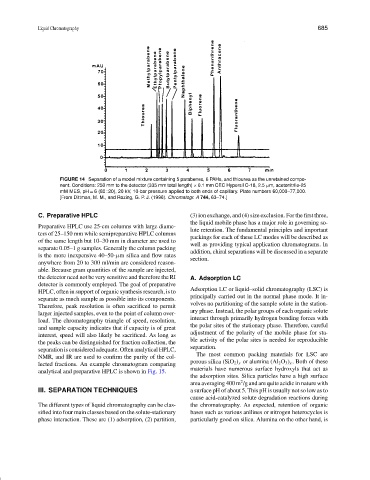
FIGURE 14 Separation of a model mixture containing 5 parabenes, 6 PAHs, and thiourea as the unretained compo-
nent. Conditions: 250 mm to the detector (335 mm total length) × 0.1 mm CEC Hypersil C-18, 2.5 µm, acetonitrile-25
mM MES, pH = 6 (80 : 20), 20 kV, 10-bar pressure applied to both ends of capillary. Plate numbers 60,000–77,000.
[From Dittman, M. M., and Rozing, G. P. J. (1996). Chromatogr. A 744, 63–74.]
C. Preparative HPLC (3) ion exchange, and (4) size exclusion. For the first three,
the liquid mobile phase has a major role in governing so-
Preparative HPLC use 25-cm columns with large diame-
lute retention. The fundamental principles and important
ters of 25–150 mm while semipreparative HPLC columns
packings for each of these LC modes will be described as
of the same length but 10–30 mm in diameter are used to
well as providing typical application chromatograms. In
separate 0.05–1 g samples. Generally the column packing
addition, chiral separations will be discussed in a separate
is the more inexpensive 40–50-µm silica and flow rates section.
anywhere from 20 to 300 ml/min are considered reason-
able. Because gram quantities of the sample are injected,
the detector need not be very sensitive and therefore the RI A. Adsorption LC
detector is commonly employed. The goal of preparative
Adsorption LC or liquid–solid chromatography (LSC) is
HPLC, often in support of organic synthesis research, is to
principally carried out in the normal phase mode. It in-
separate as much sample as possible into its components.
volves no partitioning of the sample solute in the station-
Therefore, peak resolution is often sacrificed to permit
ary phase. Instead, the polar groups of each organic solute
larger injected samples, even to the point of column over-
interact through primarily hydrogen bonding forces with
load. The chromatography triangle of speed, resolution,
the polar sites of the stationary phase. Therefore, careful
and sample capacity indicates that if capacity is of great
adjustment of the polarity of the mobile phase for sta-
interest, speed will also likely be sacrificed. As long as
ble activity of the polar sites is needed for reproducible
the peaks can be distinguished for fraction collection, the
separation.
separation is considered adequate. Often analytical HPLC,
The most common packing materials for LSC are
NMR, and IR are used to confirm the purity of the col-
porous silica (SiO 2 ) x or alumina (Al 2 O 3 ) x . Both of these
lected fractions. An example chromatogram comparing
materials have numerous surface hydroxyls that act as
analytical and preparative HPLC is shown in Fig. 15.
the adsorption sites. Silica particles have a high surface
2
area averaging 400 m /g and are quite acidic in nature with
III. SEPARATION TECHNIQUES a surface pH of about 5. This pH is usually not so low as to
cause acid-catalyzed solute degradation reactions during
The different types of liquid chromatography can be clas- the chromatography. As expected, retention of organic
sified into four main classes based on the solute-stationary bases such as various anilines or nitrogen heterocycles is
phase interaction. These are (1) adsorption, (2) partition, particularly good on silica. Alumina on the other hand, is