Page 341 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 341
P1: GNH/FEE P2: GPJ Final Pages
Encyclopedia of Physical Science and Technology EN010C-493 July 19, 2001 20:30
706 Nuclear Magnetic Resonance (NMR)
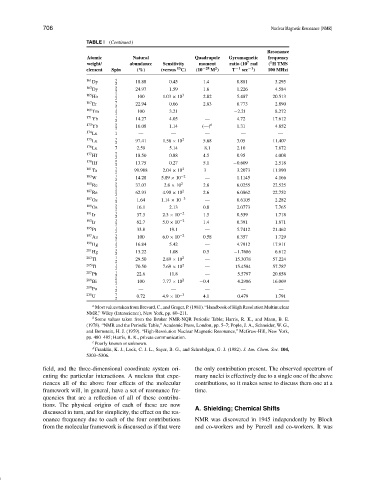
TABLE I (Continued )
Resonance
Atomic Natural Quadrupole Gyromagnetic frequency
7
1
weight/ abundance Sensitivity moment ratio (10 rad ( H TMS
2
element Spin (%) (versus 13 C) (10 −28 M ) T −1 sec −1 ) 100 MHz)
161 5
Dy 18.88 0.45 1.4 0.881 3.295
2
163 Dy 5 2 24.97 1.59 1.6 1.226 4.584
165 Ho 7 100 1.03 × 10 3 2.82 5.487 20.513
2
167 Er 7 22.94 0.66 2.83 0.773 2.890
2
169 1
Tm 100 3.21 — −2.21 8.272
2
171 Yb 1 14.27 4.05 — 4.72 17.612
2
173 Yb 5 16.08 1.14 (—) b 1.31 4.852
2
174 Lu 1 — — — — —
175 Lu 7 97.41 1.56 × 10 2 5.68 3.05 11.407
2
176 Lu 7 2.59 5.14 8.1 2.10 7.872
177 Hf 7 18.50 0.88 4.5 0.95 4.008
2
179 9
Hf 13.75 0.27 5.1 −0.609 2.518
2
181 Ta 7 99.988 2.04 × 10 2 3 3.2073 11.990
2
183 W 1 14.28 5.89 × 10 −2 — 1.1145 4.166
2
185 Re 5 37.07 2.8 × 10 2 2.8 6.0255 22.525
2
187 5 2
Re 62.93 4.90 × 10 2.6 6.0862 22.752
2
187 1 −3
Os 1.64 1.14 × 10 — 0.6105 2.282
2
189 Os 3 2 16.1 2.13 0.8 2.0773 7.765
191 Ir 3 37.3 2.3 × 10 −2 1.5 0.539 1.718
2
193 Ir 3 62.7 5.0 × 10 −2 1.4 0.391 1.871
2
195 1
Pt 33.8 19.1 — 5.7412 21.462
2
197 Au 3 100 6.0 × 10 −2 0.58 0.357 1.729
2
199 Hg 1 16.84 5.42 — 4.7912 17.911
2
201 Hg 3 13.22 1.08 0.5 −1.7686 6.612
2
203 1 2
Tl 29.50 2.89 × 10 — 15.3078 57.224
2
205 1 2
Tl 70.50 7.69 × 10 — 15.4584 57.787
2
207 Pb 1 2 22.6 11.8 — 5.5797 20.858
209 Bi 9 100 7.77 × 10 2 −0.4 4.2986 16.069
2
209 Po 1 — — — — —
2
235 7 −3
U 0.72 4.9 × 10 4.1 0.479 1.791
2
a
MostvaluestakenfromBrevard,C.,andGrager,P.(1981).“HandbookofHighResolutionMultinuclear
NMR,” Wiley (Interscience), New York, pp. 80–211.
b
Some values taken from the Bruker NMR-NQR Periodic Table; Harris, R. K., and Mann, B. E.
(1978). “NMR and the Periodic Table,” Academic Press, London, pp. 5–7; Pople, J. A., Schneider, W. G.,
and Bernstein, H. J. (1959). “High-Resolution Nuclear Magnetic Resonance,” McGraw-Hill, New York,
pp. 480–485; Harris, R. K., private communication.
c
Poorly known or unknown.
d Franklin, K. J., Lock, C. J. L., Sayer, B. G., and Schrobilgen, G. J. (1982). J. Am. Chem. Soc. 104,
5303–5306.
field, and the three-dimensional coordinate system ori- the only contribution present. The observed spectrum of
enting the particular interactions. A nucleus that expe- many nuclei is effectively due to a single one of the above
riences all of the above four effects of the molecular contributions, so it makes sense to discuss them one at a
framework will, in general, have a set of resonance fre- time.
quencies that are a reflection of all of these contribu-
tions. The physical origins of each of these are now
A. Shielding; Chemical Shifts
discussed in turn, and for simplicity, the effect on the res-
onance frequency due to each of the four contributions NMR was discovered in 1945 independently by Bloch
from the molecular framework is discussed as if that were and co-workers and by Purcell and co-workers. It was