Page 346 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 346
P1: GNH/FEE P2: GPJ Final Pages
Encyclopedia of Physical Science and Technology EN010C-493 July 19, 2001 20:30
Nuclear Magnetic Resonance (NMR) 711
of the lifetime of the states involved in the transition, the further example of the use of NMR to elucidate the struc-
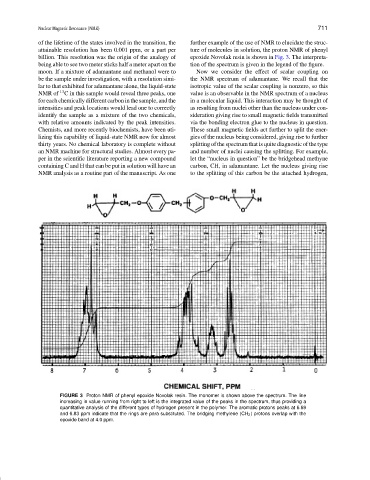
attainable resolution has been 0.001 ppm, or a part per ture of molecules in solution, the proton NMR of phenyl
billion. This resolution was the origin of the analogy of epoxide Novolak resin is shown in Fig. 3. The interpreta-
being able to see two meter sticks half a meter apart on the tion of the spectrum is given in the legend of the figure.
moon. If a mixture of adamantane and methanol were to Now we consider the effect of scalar coupling on
be the sample under investigation, with a resolution simi- the NMR spectrum of adamantane. We recall that the
lar to that exhibited for adamantane alone, the liquid-state isotropic value of the scalar coupling is nonzero, so this
13
NMR of C in this sample would reveal three peaks, one value is an observable in the NMR spectrum of a nucleus
for each chemically different carbon in the sample, and the in a molecular liquid. This interaction may be thought of
intensities and peak locations would lead one to correctly as resulting from nuclei other than the nucleus under con-
identify the sample as a mixture of the two chemicals, sideration giving rise to small magnetic fields transmitted
with relative amounts indicated by the peak intensities. via the bonding electron glue to the nucleus in question.
Chemists, and more recently biochemists, have been uti- These small magnetic fields act further to split the ener-
lizing this capability of liquid-state NMR now for almost gies of the nucleus being considered, giving rise to further
thirty years. No chemical laboratory is complete without splitting of the spectrum that is quite diagnostic of the type
an NMR machine for structural studies. Almost every pa- and number of nuclei causing the splitting. For example,
per in the scientific literature reporting a new compound let the “nucleus in question” be the bridgehead methyne
containing C and H that can be put in solution will have an carbon, CH, in adamantane. Let the nucleus giving rise
NMR analysis as a routine part of the manuscript. As one to the splitting of this carbon be the attached hydrogen,
FIGURE 3 Proton NMR of phenyl epoxide Novolak resin. The monomer is shown above the spectrum. The line
increasing in value running from right to left is the integrated value of the peaks in the spectrum, thus providing a
quantitative analysis of the different types of hydrogen present in the polymer. The aromatic protons peaks at 6.69
and 6.83 ppm indicate that the rings are para substituted. The bridging methylene (CH 2 ) protons overlap with the
epoxide band at 4.0 ppm.