Page 422 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 422
P1: GLQ Final pages
Encyclopedia of Physical Science and Technology EN012C-568 July 26, 2001 15:32
72 Photoelectron Spectroscopy
long as the temperature of the sample is not much higher and initial state. For example, the first ionization band of
than 300 K, vibrational excitations in the initial states do formaldehyde (Fig. 1b) shows a very intense 0-0 transi-
not perturb the observed spectra; low-frequency vibrations tion and little vibrational fine structure. From this we can
excited at these temperatures are covered by the limited conclude that the electronic ground state of the formalde-
resolution. hyde cation is very similar in geometry to the electronic
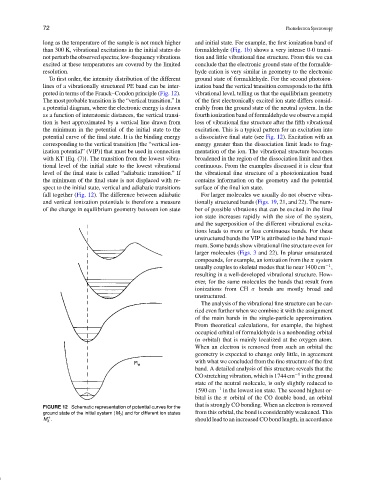
To first order, the intensity distribution of the different ground state of formaldehyde. For the second photoion-
lines of a vibrationally structured PE band can be inter- ization band the vertical transition corresponds to the fifth
preted in terms of the Franck–Condon principle (Fig. 12). vibrational level, telling us that the equilibrium geometry
The most probable transition is the “vertical transition.” In of the first electronically excited ion state differs consid-
a potential diagram, where the electronic energy is drawn erably from the ground state of the neutral system. In the
as a function of interatomic distances, the vertical transi- fourth ionization band of formaldehyde we observe a rapid
tion is best approximated by a vertical line drawn from loss of vibrational fine structure after the fifth vibrational
the minimum in the potential of the initial state to the excitation. This is a typical pattern for an excitation into
potential curve of the final state. It is the binding energy a dissociative final state (see Fig. 12). Excitation with an
corresponding to the vertical transition [the “vertical ion- energy greater than the dissociation limit leads to frag-
ization potential” (VIP)] that must be used in connection mentation of the ion. The vibrational structure becomes
with KT [Eq. (7)]. The transition from the lowest vibra- broadened in the region of the dissociation limit and then
tional level of the initial state to the lowest vibrational continuous. From the examples discussed it is clear that
level of the final state is called “adiabatic transition.” If the vibrational fine structure of a photoionization band
the minimum of the final state is not displaced with re- contains information on the geometry and the potential
spect to the initial state, vertical and adiabatic transitions surface of the final ion state.
fall together (Fig. 12). The difference between adiabatic For larger molecules we usually do not observe vibra-
and vertical ionization potentials is therefore a measure tionally structured bands (Figs. 19, 21, and 22). The num-
of the change in equilibrium geometry between ion state ber of possible vibrations that can be excited in the final
ion state increases rapidly with the size of the system,
and the superposition of the different vibrational excita-
tions leads to more or less continuous bands. For these
unstructured bands the VIP is attributed to the band maxi-
mum. Some bands show vibrational fine structure even for
larger molecules (Figs. 3 and 22). In planar unsaturated
compounds, for example, an ionization from the π system
−1
usually couples to skeletal modes that lie near 1400 cm ,
resulting in a well-developed vibrational structure. How-
ever, for the same molecules the bands that result from
ionizations from CH σ bonds are mostly broad and
unstructured.
The analysis of the vibrational fine structure can be car-
ried even further when we combine it with the assignment
of the main bands in the single-particle approximation.
From theoretical calculations, for example, the highest
occupied orbital of formaldehyde is a nonbonding orbital
(n orbital) that is mainly localized at the oxygen atom.
When an electron is removed from such an orbital the
geometry is expected to change only little, in agreement
with what we concluded from the fine structure of the first
band. A detailed analysis of this structure reveals that the
CO stretching vibration, which is 1744 cm −1 in the ground
state of the neutral molecule, is only slightly reduced to
1590 cm −1 in the lowest ion state. The second highest or-
bital is the π orbital of the CO double bond, an orbital
that is strongly CO bonding. When an electron is removed
FIGURE 12 Schematic representation of potential curves for the
ground state of the initial system (M 0 ) and for different ion states from this orbital, the bond is considerably weakened. This
+
M . should lead to an increased CO bond length, in accordance
k