Page 67 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 67
P1: GKD/LOW Revised Pages P2: FPP
Encyclopedia of Physical Science and Technology en001f-44 May 7, 2001 15:8
Auger Electron Spectroscopy 791
FIGURE 4 The change in the ductile to brittle transition temper-
ature plotted as a function of the grain boundary concentration of
either phosphorus or antimony. The steels were a Ni-Cr base ma-
terial and were doped with one of these two elements. The grain
boundary concentration was determined by Auger electron spec-
troscopy. [From Mulford, R. A., McMahon, C. J., Jr., Pope, D. P.,
and Feng, H. C. (1976). Metall. Trans. A, 7A, 1269]
caustic solutions. Furthermore, it has been well estab-
lished that segregants that embrittle grain boundaries also
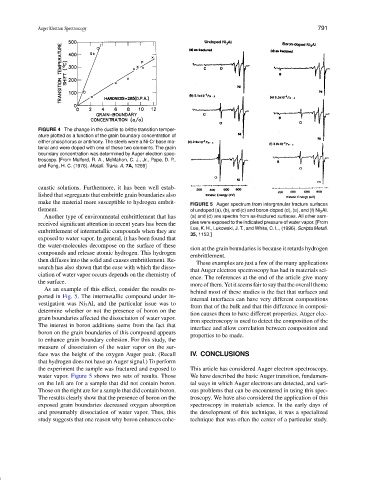
make the material more susceptible to hydrogen embrit- FIGURE 5 Auger spectrum from intergranular fracture surfaces
tlement. of undoped (a), (b), and (c) and boron-doped (d), (e), and (f) Ni 3 Al.
Another type of environmental embrittlement that has (a) and (d) are spectra from as-fractured surfaces. All other sam-
received significant attention in recent years has been the ples were exposed to the indicated pressure of water vapor. [From
Lee, K. H., Lukowski, J. T., and White, C. L., (1996). Scripta Metall.
embrittlement of intermetallic compounds when they are
35, 1153.]
exposed to water vapor. In general, it has been found that
the water-molecules decompose on the surface of these
sion at the grain boundaries is because it retards hydrogen
compounds and release atomic hydrogen. This hydrogen
embrittlement.
then diffuses into the solid and causes embrittlement. Re-
These examples are just a few of the many applications
search has also shown that the ease with which the disso-
that Auger electron spectroscopy has had in materials sci-
ciation of water vapor occurs depends on the chemistry of
ence. The references at the end of the article give many
the surface.
more of them. Yet it seems fair to say that the overall theme
As an example of this effect, consider the results re-
behind most of these studies is the fact that surfaces and
ported in Fig. 5. The intermetallic compound under in-
internal interfaces can have very different compositions
vestigation was Ni 3 Al, and the particular issue was to
from that of the bulk and that this difference in composi-
determine whether or not the presence of boron on the
tion causes them to have different properties. Auger elec-
grain boundaries affected the dissociation of water vapor.
tron spectroscopy is used to detect the composition of the
The interest in boron additions stems from the fact that
interface and allow correlation between composition and
boron on the grain boundaries of this compound appears
properties to be made.
to enhance grain boundary cohesion. For this study, the
measure of dissociation of the water vapor on the sur-
face was the height of the oxygen Auger peak. (Recall IV. CONCLUSIONS
that hydrogen does not have an Auger signal.) To perform
the experiment the sample was fractured and exposed to This article has considered Auger electron spectroscopy.
water vapor. Figure 5 shows two sets of results. Those We have described the basic Auger transition, fundamen-
on the left are for a sample that did not contain boron. tal ways in which Auger electrons are detected, and vari-
Those on the right are for a sample that did contain boron. ous problems that can be encountered in using this spec-
The results clearly show that the presence of boron on the troscopy. We have also considered the application of this
exposed grain boundaries decreased oxygen absorption spectroscopy in materials science. In the early days of
and presumably dissociation of water vapor. Thus, this the development of this technique, it was a specialized
study suggests that one reason why boron enhances cohe- technique that was often the center of a particular study.