Page 66 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 66
P1: GKD/LOW Revised Pages P2: FPP
Encyclopedia of Physical Science and Technology en001f-44 May 7, 2001 15:8
790 Auger Electron Spectroscopy
Let us first consider the external surfaces. The most in which results could be misinterpreted. One is that the
common types of experiments that are performed are those surface will be covered with enough oxygen and carbon
in which the surface is cleaned and then heated to allow from the environment to mask the surface species that may
segregation. The heating allows solid state diffusion to oc- have originally caused a problem. The second is that con-
cur, and one finds that with time the composition of the taminants from handling of the material may show up and
surface can be significantly altered as a result of this seg- mislead the researcher in his or her effort to find the root
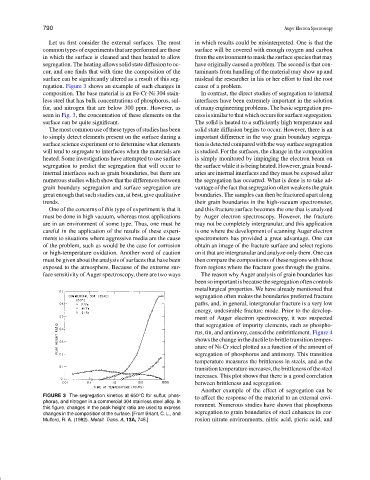
regation. Figure 3 shows an example of such changes in cause of a problem.
composition. The base material is an Fe-Cr-Ni 304 stain- In contrast, the direct studies of segregation to internal
less steel that has bulk concentrations of phosphorus, sul- interfaces have been extremely important in the solution
fur, and nitrogen that are below 300 ppm. However, as of many engineering problems. The basic segregation pro-
seen in Fig. 3, the concentration of these elements on the cess is similar to that which occurs for surface segregation.
surface can be quite significant. The solid is heated to a sufficiently high temperature and
The most common use of these types of studies has been solid state diffusion begins to occur. However, there is an
to simply detect elements present on the surface during a important difference in the way grain boundary segrega-
surface science experiment or to determine what elements tionisdetectedcomparedwiththewaysurfacesegregation
will tend to segregate to interfaces when the materials are is studied. For the surfaces, the change in the composition
heated. Some investigations have attempted to use surface is simply monitored by impinging the electron beam on
segregation to predict the segregation that will occur to the surface while it is being heated. However, grain bound-
internal interfaces such as grain boundaries, but there are aries are internal interfaces and they must be exposed after
numerous studies which show that the differences between the segregation has occurred. What is done is to take ad-
grain boundary segregation and surface segregation are vantageofthefactthatsegregationoftenweakensthegrain
great enough that such studies can, at best, give qualitative boundaries. The samples can then be fractured apart along
trends. their grain boundaries in the high-vacuum spectrometer,
One of the concerns of this type of experiment is that it and this fracture surface becomes the one that is analyzed
must be done in high vacuum, whereas most applications by Auger electron spectroscopy. However, the fracture
are in an environment of some type. Thus, one must be may not be completely intergranular, and this application
careful in the application of the results of these experi- is one where the development of scanning Auger electron
ments to situations where aggressive media are the cause spectrometers has provided a great advantage. One can
of the problem, such as would be the case for corrosion obtain an image of the fracture surface and select regions
or high-temperature oxidation. Another word of caution on it that are intergranular and analyze only them. One can
must be given about the analysis of surfaces that have been then compare the compositions of these regions with those
exposed to the atmosphere. Because of the extreme sur- from regions where the fracture goes through the grains.
face sensitivity of Auger spectroscopy, there are two ways The reason why Auger analysis of grain boundaries has
been so important is because the segregation often controls
metallurgical properties. We have already mentioned that
segregation often makes the boundaries preferred fracture
paths, and, in general, intergranular fracture is a very low
energy, undesirable fracture mode. Prior to the develop-
ment of Auger electron spectroscopy, it was suspected
that segregation of impurity elements, such as phospho-
rus, tin, and antimony, caused the embrittlement. Figure 4
shows the change in the ductile to brittle transition temper-
ature of Ni-Cr steel plotted as a function of the amount of
segregation of phosphorus and antimony. This transition
temperature measures the brittleness in steels, and as the
transition temperature increases, the brittleness of the steel
increases. This plot shows that there is a good correlation
between brittleness and segregation.
Another example of the effect of segregation can be
FIGURE 3 The segregation kinetics at 650 C for sulfur, phos- to affect the response of the material to an external envi-
◦
phorus, and nitrogen in a commercial 304 stainless steel alloy. In
this figure, changes in the peak height ratio are used to express ronment. Numerous studies have shown that phosphorus
changes in the composition of the surface. [From Briant, C. L., and segregation to grain boundaries of steel enhances its cor-
Mulford, R. A. (1982). Metall. Trans. A, 13A, 745.] rosion nitrate environments, nitric acid, picric acid, and