Page 167 - Academic Press Encyclopedia of Physical Science and Technology 3rd BioChemistry
P. 167
P1: GPAFinal Pages
Encyclopedia of Physical Science and Technology EN013D-616 July 27, 2001 12:5
Protein Structure 207
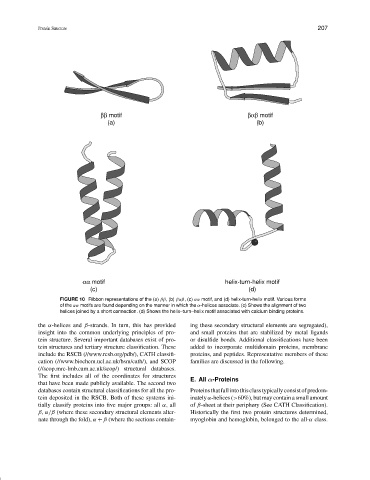
ββ motif βαβ motif
(a) (b)
αα motif helix-turn-helix motif
(c) (d)
FIGURE 10 Ribbon representations of the (a) ββ, (b) βαβ, (c) αα motif, and (d) helix-turn-helix motif. Various forms
of the αα motifs are found depending on the manner in which the α-helices associate. (c) Shows the alignment of two
helices joined by a short connection. (d) Shows the helix–turn–helix motif associated with calcium binding proteins.
the α-helices and β-strands. In turn, this has provided ing these secondary structural elements are segregated),
insight into the common underlying principles of pro- and small proteins that are stabilized by metal ligands
tein structure. Several important databases exist of pro- or disulfide bonds. Additional classifications have been
tein structures and tertiary structure classification. These added to incorporate multidomain proteins, membrane
include the RSCB (//www.rcsb.org/pdb/), CATH classifi- proteins, and peptides. Representative members of these
cation (//www.biochem.ucl.ac.uk/bsm/cath/), and SCOP families are discussed in the following.
(//scop.mrc-lmb.cam.ac.uk/scop/) structural databases.
The first includes all of the coordinates for structures
E. All α-Proteins
that have been made publicly available. The second two
databases contain structural classifications for all the pro- Proteinsthatfallintothisclasstypicallyconsistofpredom-
tein deposited in the RSCB. Both of these systems ini- inately α-helices (>60%), but may contain a small amount
tially classify proteins into five major groups: all α, all of β-sheet at their periphery (See CATH Classification).
β, α/β (where these secondary structural elements alter- Historically the first two protein structures determined,
nate through the fold), α + β (where the sections contain- myoglobin and hemoglobin, belonged to the all-α class.