Page 175 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 175
P1: GKY/MBQ P2: GLQ Final Pages
Encyclopedia of Physical Science and Technology en012i-947 July 26, 2001 11:11
Polymers, Inorganic and Organometallic 683
canexceed15 000.Ifthelinearpolymerisheatedfurtheror
at higher temperatures, a crosslinked elastomeric polymer
is formed.
The high reactivity of the P Cl bond toward nucle-
ophilic substitution provides a facile route to polymers
with a variety of organic, organometallic and inorganic
substituents. For example, homopolymers [Eq. (19)] and
copolymers [Eq. (20)] are available through this route:
Cl OR
(19)
N P 6n NaOR N P 6 NaCl
3n 3n
Cl OR
Cl OR OR
3n NaOR 3n RNH 2 (20)
N P N P N P
3n 3 NaCl 3n 3 HCl 3n
FIGURE 19 Synthesis of linear polyphosphazenes via noncyclic
Cl Cl NHR
intermediates.
Halogen substitution reactions on linear polymers
sometimes suffer from incomplete halogen replacement or
ter. Polymers generated by this process are often difficult
unwanted chain scission. These problems are sometimes
to obtain by other means.
precluded by ROP of cyclic trimers that have one or more
Polyphosphazenes are also prepared using “living”
of the halogens replaced with R groups [Eq. (21)]. This
cationic catalysts at room temperature [Eq. (23)]. This
modified approach controls the substitutent distribution.
direct synthesis from monomer to polymer allows control
Although polymers have been obtained with several dif-
over polymer architecture, MW, polydispersity, and block
ferent organic groups attached, polymerization becomes
copolymer composition. It has also been used to prepare
more difficult as the number of groups increase. It is
star-branched polymers and organic PN hybrid copoly-
noteworthy that polymerization is more facile if the sub-
mers. The active initiator in this synthesis is believed to be
stituents cause steric ring strain as in the ferrocenyl phos-
+
−
theshort-chainionicintermediate[Cl 3 P NPCl] [PCl 6 ] .
phazene trimer [Eq. (22)]:
Cl OR
Cl Cl Cl R 1. PCl 5
(CH 3 ) 3 Si N P Cl N P
P P 2. NaOR n (23)
N N 3 RM N N Cl OR
Cl Cl R R
3 MCl
P P P P Polyphosphazenes have been prepared with MW from
Cl N Cl Cl N Cl
several thousand to several million. Although the formula
1. Heat
2. NaOR′ is usually written as alternating single and double bonds
(21) ( P N P N ), all the P N lengths are approximately
R
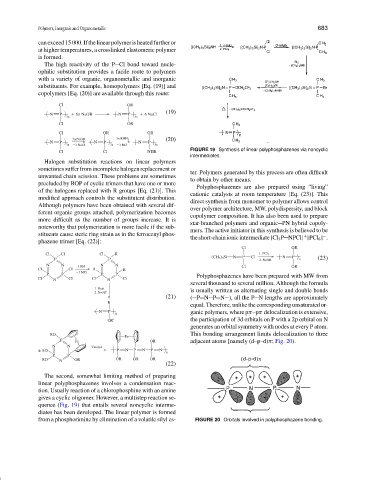
equal. Therefore, unlike the corresponding unsaturated or-
N P ganic polymers, where pπ–pπ delocalization is extensive,
3n
OR′ the participation of 3d orbitals on P with a 2p orbital on N
generates an orbital symmetry with nodes at every P atom.
RO This bonding arrangement limits delocalization to three
Fe
P Fe OR adjacent atoms [namely (d–p–d)π; Fig. 20).
N N Catalyst
n RO P N P N P N n
P P
RO N OR OR OR OR
(22)
The second, somewhat limiting method of preparing
linear polyphosphazenes involves a condensation reac-
tion. Usually reaction of a chlorophosphine with an amine
gives a cyclic oligomer. However, a multistep reaction se-
quence (Fig. 19) that entails several noncyclic interme-
diates has been developed. The linear polymer is formed
from a phosphorimine by elimination of a volatile silyl es- FIGURE 20 Orbitals involved in polyphosphazene bonding.